��Ŀ����
10����A��B��C��D��E���ֶ�����Ԫ�أ����ǵĺ˵������C��A��B��D��E��˳������C��D���ֱܷ���A��ԭ�Ӹ�����Ϊ1��1��2��1�γɻ����CB����EA2��Ӧ����C2A����̬����EB4��E��M���������K���������2������1��д������Ԫ�ص����ƣ�A����B����E�裮
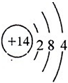
��2������E��ԭ�ӽṹʾ��ͼ
 ��
����3���Ƚ�EA2��EB4���۵�ߵͣ��ѧʽ��SiO2��SiF4��
��4��A���⻯��ķе���ͬ����һ����Ԫ�ص��⻯����ȣ�A���⻯��ķе�ߣ���ߡ��͡�����ԭ����ˮ����֮���γ��������H2S�в����������
��5��д������ʽD2A2
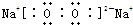 ��EB4
��EB4 ��
��
���� A��B��C��D��E���ֶ�����Ԫ�أ��˵������C��A��B��D��E��˳������E��M���������K���������2����K������2�����ӣ�M����4�����ӣ���EΪSiԪ�أ�C��D���ֱܷ���A��ԭ�Ӹ�����Ϊ1��1��2��1�γɻ������CΪHԪ�أ�AΪOԪ�أ�DΪNaԪ�أ�CB����EA2��Ӧ����C2A����̬����EB4���÷�ӦΪ4HF+SiO2�T2H2O+SiF4������BΪFԪ�أ��ݴ˽��
��� �⣺A��B��C��D��E���ֶ�����Ԫ�أ��˵������C��A��B��D��E��˳������E��M���������K���������2����K������2�����ӣ�M����4�����ӣ���EΪSiԪ�أ�C��D���ֱܷ���A��ԭ�Ӹ�����Ϊ1��1��2��1�γɻ������CΪHԪ�أ�AΪOԪ�أ�DΪNaԪ�أ�CB����EA2��Ӧ����C2A����̬����EB4���÷�ӦΪ4HF+SiO2�T2H2O+SiF4������BΪFԪ�أ�
��1��������������֪��AΪ����BΪ����EΪ�裬
�ʴ�Ϊ�����������裻
��2��EΪSiԪ�أ�������Ϊ14������������Ϊ4��ԭ�ӽṹʾ��ͼΪ ��
��
�ʴ�Ϊ�� ��
��
��3��EA2��SiO2��EB4��SiF4��SiO2Ϊԭ�Ӿ��壬�۷е�ϸߣ�SiF4Ϊ���Ӿ��壬�۷е�ϵͣ��е�SiO2��SiF4��
�ʴ�Ϊ��SiO2��SiF4��
��4��AΪOԪ�أ���Ӧ�⻯��ΪH2O����Aͬ����һ����Ԫ�ص��⻯��ΪH2S������OԪ�صĵ縺�Ժ�ǿ��ˮ����֮���γ��������H2S�в��������������ˮ�ķе��H2S�ķе�ߣ�
�ʴ�Ϊ���ߣ�ˮ����֮���γ��������H2S�в����������
��5��D2A2ΪNa2O2��������������������ӹ��ɣ�����ʽΪ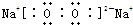 ��
��
EB4ΪSiF4����ԭ�����ԭ��֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ ��
��
�ʴ�Ϊ��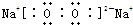 ��
�� ��
��
���� ���⿼��ṹ����λ�ù�ϵ�ۺ�Ӧ�ã���ϳ���������Ľṹ�ƶ�Ԫ���ǽ����Ĺؼ���ע�������������ԣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | H2+Cl2$\stackrel{����}{��}$2HCl | B�� | NH4Cl$\stackrel{����}{��}$NH3��+HCl�� | ||
| C�� | CH4+Cl2$\stackrel{����}{��}$CH3Cl+HCl | D�� | 2NaCl+H2SO4��Ũ��=Na2SO4+2HCl�� |
| A�� | NaOH | B�� | Na2O2 | C�� | CaCl2 | D�� | H2O2 |
| A�� | �����CO2��NO��SO2�� | B�� | �NaOH��KOH��Na2CO3 | ||
| C�� | ��Σ�NH4Cl��NH4NO3��NH3•H2O | D�� | ���������Na2O��CaO��Al2O3 |
| A�� | �Ȼ�����ԭ�Ӿ��� | |
| B�� | �ۻ�ʱ���Ȼ����ܵ��� | |
| C�� | �Ȼ�����һ�����Ӿ��� | |
| D�� | ˮ�ⷽ��ʽ��BCl3+3H2O?H3BO3+3HCl |
| A�� | ʵ�����ô���ʯ����ᷴӦ��ȡ������̼��CaCO3+2H+=Ca2++CO2��+H2O | |
| B�� | ����������������������Һ���ȣ�CH2ClCOOH+OH-��CH2ClCOO-+H2O | |
| C�� | ��������Һ��ͨ������������̼��2C6H5O-+CO2+H2O��2C6H5OH+CO32- | |
| D�� | ������Cu��OH��2������ȩ�е�ȩ����CH3CHO+2Cu��OH��2+NaOH $\stackrel{��}{��}$CH3COONa+Cu2O��+3H2O |
| A�� | $\frac{m+n+2}{w}$mol | B�� | $\frac{m-n+2}{m}$ mol | C�� | $\frac{m+n-2}{m}$ mol | D�� | $\frac{W��m-n-2��}{m}$mol |

 ����������ˮ�����ڹ��ۼ��ܵ��ƻ��������H+��Q-��ʹ��Һ�����ԣ��ṹ������ͬY��W���⻯��ķе�Ӹߵ������д����ǣ��ѧʽ��H2O��H2S��
����������ˮ�����ڹ��ۼ��ܵ��ƻ��������H+��Q-��ʹ��Һ�����ԣ��ṹ������ͬY��W���⻯��ķе�Ӹߵ������д����ǣ��ѧʽ��H2O��H2S��