��Ŀ����
����Ŀ����1��������ۻ������Ӽ�˷���________�������������������ۻ������Ӽ�˷���________����������������������Ӽ�˷���________�������������־�����۵��ɸߵ��͵�˳����______���ѧʽ����
��2���������־��壺��CO2����NaCl����Na����Si����CS2�����ʯ�����ǵ��۵�ӵ͵��ߵ�˳��Ϊ________������ţ���
��3����H2����NH4��2SO4��SiC��CO2��HF�У��ɼ��Լ��γɵķǼ��Է�����________���ɷǼ��Լ��γɵķǼ��Է�����________�����γɷ��Ӿ����������________����������ľ���Ļ�ѧʽ��__________���������Ӿ������_________������ԭ�Ӿ������_______���������ʵ��۵��ɸߵ��͵�˳����__________��
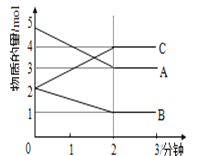
��4��A��B��C��DΪ���־��壬�������£�
A ��̬ʱ�ܵ��磬����������
B ������CS2��������ˮ
C ��̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ
D ��̬��Һ̬ʱ�������磬�۵�Ϊ3 500��
���ƶ����ǵľ������ͣ�
A��______��B.______��C��________��D._____��
��5����ͬѹǿ�£�����Ԫ�ط�������۵���±���
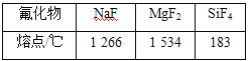
�Խ����ϱ��з������۵�����ԭ��__________
��6��������CO�е��¼��ȣ�������ɫ�ӷ���Һ̬Ni��CO��4���������幹�͡�150 ��ʱ��Ni��CO��4�ֽ�ΪNi��CO��Ni��CO����________���壬Ni��CO��4����������________������ţ�
a ˮ������b ���Ȼ�̼������c ��������d ��������Һ
���𰸡����Ӽ� ���ۼ� ���Ӽ� SiO2��KClO3��I2 ��<��<��<��<��<�� CO2 H2 H2��CO2��HF HF ��NH4��2SO4 SiC SiC����NH4��2SO4��HF��CO2��H2 �������� ���Ӿ��� ���Ӿ��� ԭ�Ӿ��� NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2���İ뾶��Na���İ뾶С��Mg2����2����λ���������Na���࣬��MgF2���۵��NaF�� ���Ӿ��� bc
��������
(1)������������Ӿ����ۻ�ʱ�ƻ����Ӽ�������������ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ������Ƿ��Ӿ��壬����ʱ���Ӽ�˷����Ӽ����������۵㣺ԭ�Ӿ���>���Ӿ���>���Ӿ��壻
(2)��һ����˵�������۵�Ϊ��ԭ�Ӿ���>���Ӿ���>���Ӿ��壻ԭ�Ӿ����У�����Խ�̣�����Խǿ���۵�Խ�ߣ����Ӿ����У��ṹ���Ƶ����ʣ���Է�������Խ���۵�Խ�ߣ��Ƶ��۵�ϵͣ�
(3)���ɲ�ͬ��ԭ���γɵĹ��ۼ����м��Թ��ۼ�����ͬ��ԭ���γɵĹ��ۼ����зǼ��Թ��ۼ�����������������������غ�ʱ�������ǷǼ��Է��ӣ�����Ϊ���Է��ӣ�
(4)�����������д��������ƶ��ĵ��ӣ����Ե��磬���ý������Ժ��ᷴӦ������̼�ǷǼ����ܼ���ˮ�Ǽ����ܼ���������������ԭ���ж�B����̬�����磬˵���������ƶ������ӻ���ӣ�Һ̬ʱ�ɵ��磬˵������ʱ�������ƶ������ӣ�����������ɣ���̬��Һ̬�������磬˵���������ƶ������ӣ��۵�ߣ�������ԭ�Ӿ��壻
(5)�������۷е��һ�������ԭ�Ӿ���>���Ӿ���>��������>���Ӿ��壻
(6)�����Ӿ�����۷е�ϵͣ����ݸ����ʵ��۷е�ȷ���������ͣ�������������ԭ��ȷ�����ܽ��ԡ�
(1)������������Ӿ����ۻ�ʱ�ƻ����Ӽ�������������ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ������Ƿ��Ӿ��壬����ʱ���Ӽ�˷����Ӽ����������۵㣺ԭ�Ӿ���>���Ӿ���>���Ӿ��壻�����۵��С˳��Ϊ��SiO2��KClO3��I2���ʴ�Ϊ�����Ӽ������ۼ������Ӽ䣻SiO2��KClO3��I2��
(2)�����ݾ������ͷ�����ԭ�Ӿ���>���Ӿ���>���Ӿ��壬Si�ͽ��ʯ����ԭ�Ӿ��壬ԭ�Ӱ뾶ԽС�����ۼ�Խǿ���۵�Խ�ߣ�CO2��CS2���Ƿ��Ӿ��壬��Է�������Խ���۵�Խ�ߣ����ǽ������壬�����۵�ϵͣ��ʴ�Ϊ����<��<��<��<��<�ޣ�
(3)���ɼ��Լ��γɵķǼ��Է�����CO2���ɷǼ��Լ��γɵķǼ��Է�����H2�����γɷ��Ӿ����������H2��CO2��HF����������ľ���Ļ�ѧʽ��HF���������Ӿ������(NH4)2SO4������ԭ�Ӿ������SiC���ʴ�Ϊ��CO2��H2�� H2��CO2��HF��HF��(NH4��2SO4��SiC��SiC����NH4��2SO4��HF��CO2��H2��
(4)���ݾ�����������ʷ�����A����̬ʱ�ܵ��磬���������ᣬ˵���ǻ��ý��������ڽ������壻
B��������CS2��������ˮ������̼�ǷǼ����ܼ���ˮ�Ǽ����ܼ��������������ܿ�֪�����ڷ��Ӿ��壻
C����̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ��˵������ˮ�����˵��룬Һ̬���Ե��磬˵������������ɵģ��������Ӿ��壻
D����̬��Һ̬ʱ�������磬�۵�Ϊ3500C���۵�ϸߣ����Ҳ�����ֻ���ƶ������ӣ�����ԭ�Ӿ��壻
�ʴ�Ϊ���������壻���Ӿ��壻���Ӿ��壻ԭ�Ӿ��壻
(5)�����Ӿ�����۷е�������Ӿ��壬�ϱ��з������۵�����ԭ���ǣ�NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2+�İ뾶��Na+�İ뾶С��Mg2+�����������Na+�࣬��MgF2���۵��NaF�ߣ��ʴ�Ϊ��NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2���İ뾶��Na���İ뾶С��Mg2����2����λ���������Na���࣬��MgF2���۵��NaF�ߣ�
(6)�����ݸ����ʵ��۷е�֪�����������ڷ��Ӿ��壬�����ʵĽṹΪ��������ṹ��������������غϣ�Ϊ�Ǽ��Է��ӣ�������������ԭ��֪���Ǽ��Է��ӵ����������ڷǼ��Է��ӵ��ܼ����������Ȼ�̼���ǷǼ��Է��ӣ����Ը����������ڱ������Ȼ�̼���ʴ�Ϊ�����Ӿ��壻bc��

����Ŀ������ijʵ��С���H2O2�ķֽ���������̽�����±��Ǹ�ʵ��С���о�Ӱ��H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ���������ͬ�ķ�ĩ״�Ϳ�״��MnO2�ֱ����ʢ��15 ml 5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�������£�
MnO2 | �����Թ���� | �۲��� | ��Ӧ��������ʱ�� |
��ĩ״ | ���� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
��״ | �� | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��1��д������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��______��
��2��ʵ���������������Ĵ�Ч����_____�йء�
��3��ijͬѧ��10 mL H2O2 ��Һ�м���һ�����Ķ������̣��ų�������������״�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����A��B��C��������ʾ�ķ�Ӧ������������_____��

����ij��Ӧ�����Ϊ5L�ĺ����ܱ������н��У� ��0-3�����ڸ����ʵ����ı仯�����ͼ��ʾ��A��B��C��Ϊ���壬��A��������ɫ����
��4���÷�Ӧ�ĵĻ�ѧ����ʽΪ__________��
��5����Ӧ��ʼ��2����ʱ��B��ƽ����Ӧ����Ϊ____��
��6����˵���÷�Ӧ�Ѵﵽƽ��״̬����____��
a��v(A)= 2v(B) b�������ڸ����ʵ����ʵ������
c��v��(A)=v��(C) d���������������ɫ���ֲ���
��7����ͼ���ƽ��ʱA���������______��