��Ŀ����
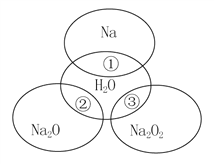
����Ŀ��ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪2 mol��ɫ�����ĩX���ȷֽ⣬�ָ����������ɰ�ɫ����A����ɫҺ��B����ɫ����C��1 mol��X��E��G����ɫ��Ӧ��Ϊ��ɫ��

�ش��������⣺
(1)д���������ʵĻ�ѧʽ��G________��D________��
(2)д��G��C��Ӧ����D�Ļ�ѧ��Ӧ����ʽ��____________________________��
(3)д��X��E��A�����ӷ���ʽ��______________________________________��
(4)д��C��Na2O2��Ӧ�Ļ�ѧ����ʽ��_____________________________������0.2 mol Na2O2�μӷ�Ӧ����ת�Ƶĵ�����ĿΪ________��
���𰸡�NaAlO2 Al(OH)3 NaAlO2+CO2+2H2O��Al(OH)3��+NaHCO3��2NaAlO2+CO2+3H2O��2Al(OH)3��+Na2CO3 HCO3-+OH-��CO32-+H2O 2Na2O2+2CO2��2Na2CO3+O2 0.2NA��1.204��1023
��������
BΪ��ɫҺ�壬B��Na2O2��Ӧ���ɵ�E����Al��Ӧ����X��E��G����ɫΪ��ɫ���Ƴ�BΪH2O��EΪNaOH��GΪNaAlO2��C����ɫ���壬C����Na2O2��Ӧ����A��A+HCl��C����AΪNa2CO3��CΪCO2��2 mol��ɫ�����ĩX���ȷֽ⣬�ָ�����������A��B��C��1 mol��XΪNaHCO3��C��G��Ӧ����D��CO2��NaAlO2��Һ��Ӧ���ɵ�DΪAl(OH)3���ݴ˽��
��1����������������֪G�Ļ�ѧʽΪNaAlO2��D�Ļ�ѧʽΪAl(OH)3��
��2��G+C��D��CO2��NaAlO2��Һ�ķ�Ӧ����CO2��������Ӧ�Ļ�ѧ����ʽΪNaAlO2+CO2+2H2O��Al��OH��3��+NaHCO3����CO2��������Ӧ�Ļ�ѧ����ʽΪ2NaAlO2+CO2+3H2O��2Al(OH)3��+Na2CO3��
��3��X+E��A��Ӧ�Ļ�ѧ����ʽΪNaHCO3+NaOH��Na2CO3+H2O����Ӧ�����ӷ���ʽΪHCO3-+OH-��CO32-+H2O��
��4��C��Na2O2��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2��2Na2CO3+O2��2molNa2O2�μӷ�Ӧת��2mol���ӣ���0.2molNa2O2�μӷ�Ӧת��0.2mol���ӣ�ת�Ƶ�����Ϊ0.2NA��1.204��1023��