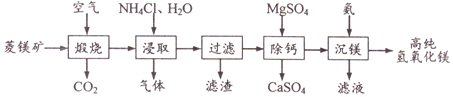
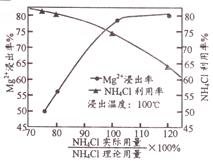
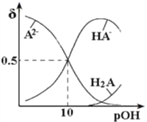
��Ŀ����
����Ŀ��С��ͬѧ��ʵ����ѡ�õ������ⶨij������(��Ҫ�ɷ��� Fe3O4������SiO2��CuO)����Ԫ������������ʵ�鲽�����£�
�������ȡ��m g���Ƚ���ʯ���飬��������2mol/LH2SO4��ȡ�����˺�õ���ȡҺ��
�ڳ��ӣ������ȡҺ�м������� H2S��Һ�����˺������������ H2O2��Һ����Fe2+(������H2Sת��ΪS)���ټ���һ����MnO2�����ȥ������H2O2��һ��ʱ�����˵õ�Vml ��Һ��
�۶��ݣ�����Һϡ����250mL��ȡ��25.00mL�ڴ����ӵ���ƿ�д��ã�
��ת������25.00mL��Һ�м�������KI���壬���������ڰ�������30min��
�ݵζ�������ָʾ������cmol/LNa2S2O3����Һ���еζ����յ㣬����Na2S2O3����Һ V1mL��
���ظ��ܢݲ���2�Ρ� ��֪��2S2O32-+I2==S4O62-+2I-
1�����н���ʯ�����Ŀ����____________________________________________���������� H2SO4��Ŀ����__________________________________��
2��д�����м������� H2S ��Һ������Ӧ�����ӷ�Ӧ����ʽ��_____________________________��
3��������______________________________ _(����������)ȡ�� 25.00mL ��Һ��
4�����м����ָʾ����________���ζ��յ�������ǣ��������һ�� Na2S2O3����Һ ��__________�������ظ�����ʵ���Ŀ����______��
5���ô���������Ԫ����������Ϊ______________���ú� m��c��V1�Ĵ���ʽ��ʾ����Ҫ����
6�����ζ���������Һ pH ������ᵼ�²ⶨ���______ (�ƫ�ߡ�����ƫ�͡�)��д�� ���������������ӷ�Ӧ����ʽ___________��
�����й����� H2O2��Һû����ᵼ�²ⶨ���_____(�ƫ�ߡ�����ƫ�͡�) ������ MnO2����û�й��˳�ȥ��ᵼ�²ⶨ���_________(�ƫ�ߡ�����ƫ�͡�)
���𰸡� �����ʯ����Һ�ĽӴ�������ӿ췴Ӧ���ʣ�ʹ Fe3O4 ��ȫ���� ʹ Fe3O4 ȫ�� ���������� Fe2+��Fe3+ˮ�� H2S+2Fe3+=S��+2H++2Fe2+��H2S+Cu2+=CuS��+2H+ ��ʽ�ζ��� ������Һ ��Һ��ɫǡ����ȥ���� 30s �ڲ���ɫ ��С��� ��56cV1/m��% ƫ�� 2H++S2O32��= H2O+S��+SO2�� ƫ�� ƫ��
��������1�����н���ʯ�����Ŀ���������ʯ����Һ�ĽӴ�������ӿ췴Ӧ���ʣ�ʹ Fe3O4 ��ȫ�������������� H2SO4��Ŀ����ʹ Fe3O4 ȫ�� ���������� Fe2+��Fe3+ˮ�⣻2�����м������� H2S ��Һ�������ӷ���������ԭ��Ӧ�������ʣ�ͬʱ����ͭ���ӷ�Ӧ����CuS������������Ӧ�����ӷ�Ӧ����ʽΪ��H2S+2Fe3+=S��+2H++2Fe2+��H2S+Cu2+=CuS��+2H+��3����������ʽ�ζ���ȷȡ�� 25.00mL�ữ����Һ��4���������۱������ʢ��м����ָʾ���ǵ�����Һ���ζ��յ�������ǣ��������һ�� Na2S2O3����Һ ����Һ��ɫǡ����ȥ���� 30s �ڲ���ɫ�������ظ�����ʵ���Ŀ���Ƕ��ʵ��ȡƽ��ֵ����С��5��ʵ��������Ƚ���ת��Ϊ�����ӣ�2Fe3++2I-=2Fe2++I2��2S2O32-+I2==S4O62-+2I-����ô���������Ԫ����������Ϊx����
2Fe3+~~~~~~~~ I2~~~~~~~~~~~2S2O32-
2��56 2
mg��x cmol/L��V1��10-3L��![]()
 ��6�����ζ���������Һ pH ������ᵼ�²ⶨ���ƫ�ߣ����������������ӷ�Ӧ����ʽΪ2H++S2O32��= H2O+S��+SO2���������й����� H2O2��Һû������������ĵⵥ�ʣ��ζ�ʱ����Na2S2O3����Һƫ�࣬�ᵼ�²ⶨ���ƫ�ߣ������� MnO2����û�й��˳�ȥ��Ҳ�Ὣ���������ⵥ�ʣ��ζ�ʱ����Na2S2O3����Һƫ�࣬�ᵼ�²ⶨ���ƫ�ߡ�
��6�����ζ���������Һ pH ������ᵼ�²ⶨ���ƫ�ߣ����������������ӷ�Ӧ����ʽΪ2H++S2O32��= H2O+S��+SO2���������й����� H2O2��Һû������������ĵⵥ�ʣ��ζ�ʱ����Na2S2O3����Һƫ�࣬�ᵼ�²ⶨ���ƫ�ߣ������� MnO2����û�й��˳�ȥ��Ҳ�Ὣ���������ⵥ�ʣ��ζ�ʱ����Na2S2O3����Һƫ�࣬�ᵼ�²ⶨ���ƫ�ߡ�