题目内容
【题目】如图所示,两圆圈相交的部分表示圆圈内的物质相互发生的反应。已知钠及其氧化物的物质的量均为0.1 mol,水的质量为100 g。下列说法正确的是( )
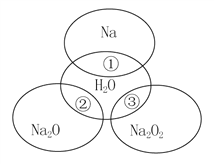
A. Na2O2中阴阳离子数目之比为1∶1
B. 反应①的离子方程式为Na+2H2O===Na++2OH-+H2↑
C. ①、②、③充分反应后所得溶液中溶质的质量分数:①>②>③
D. 反应③转移电子的物质的量为0.1mol
【答案】C
【解析】
试题A.过氧化钠中阴离子是O22-,所以阴阳离子数目之比为1:2,错误;B、反应①的离子方程式为:2Na+2H2O=2Na++2OH-+H2↑,错误;C、反应方程式为:2Na2O2+2H2O=4NaOH+O2↑转移电子2e-,则0.1mol过氧化钠反应最多能转移0.1 mol电子,正确;D、钠、氧化钠、过氧化钠和水反应的方程式分别如下:
Na+1/2H2O=NaOH+1/2H2↑,溶液增加的质量=m(Na)-m(H2)=2.3g-0.1g=2.2g;Na2O+H2O=2NaOH,溶液增加的质量=m(Na2O)=0.1mol×62g/mol=6.2g;Na2O2+H2O=2NaOH+1/2O2↑,溶液增加的质量=m(Na2O2)-m(O2)=m(Na2O)=6.2g,所以溶液增加的质量大小顺序为:钠<氧化钠=过氧化钠,根据钠原子守恒知,0.1mol的钠、氧化钠、过氧化钠、溶于水所得氢氧化钠的物质的量分别为:0.1mol、0.2mol、0.2mol,通过以上分析可知,0.1mol的钠、氧化钠、过氧化钠、分别溶于水所得溶液的质量分数分别为:4/(100+2.2)×100%、8/(100+6.2)×100%、8/(100+6.2)×100%,所以①、②、③充分反应后所得溶液的质量分数从大到小:①<②=③,错误,选C。

 阅读快车系列答案
阅读快车系列答案【题目】羰基硫(COS)常用作粮食熏蒸剂,制取反应为 CO(g)+H2S(g) ![]() COS(g)+H2(g),△H=-30kJ·mol-1。在恒容的密闭容器中不同条件下发生反应并达到平衡,数据如下表。
COS(g)+H2(g),△H=-30kJ·mol-1。在恒容的密闭容器中不同条件下发生反应并达到平衡,数据如下表。
实验 | 温度/℃ | n起始/mol | 平衡时 | 所需时间 | |||
CO | H2S | COS | H2 | n(CO)/mol | min | ||
1 | 150 | 10.0 | 10.0 | 0 | 0 | 7.0 | e |
2 | 150 | 7.0 | 7.0 | 3.0 | 3.0 | a | f |
3 | 200 | 20.0 | 20.0 | 0 | 0 | 16.0 | g |
下列说法错误的是
A. 实验1 反应开始到平衡整个过程中放出的热量为 90kJ
B. 实验2 达平衡时,a>7.0
C. 实验3 时间:g<e
D. 实验3 CO 达到平衡时的转化率比实验1 小