��Ŀ����
13��ij��ɫ��Һ��ֻ��������8�������е�ij���֣�Mg2+��H+��Ag+��Na+��Cl-��HCO3-��OH-��NO3-����֪����Һ��������Ӧ�ҷų�������ֻ���������Իش��������⣺��1������Ӧ������Al3+����Ӧ�����ӷ���ʽΪ2Al+6H+=2Al3++3H2������ԭ��Һ��һ���������ڵ�������H+��Cl-��һ�����ܴ������ڵ�������Ag+��HCO3-��OH-��NO3-��
��2������Ӧ������AlO2-����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2��������Һ��һ���������ڵ�������Na+��OH-��һ�����ܴ������ڵ�������Mg2+��H+��Ag+��HCO3-��
���� ����������Ӧ����H2����Һ����Ϊ����Һ��Ҳ����Ϊ����Һ��������Al3+ʱ����ҺΪ����Һ��������AlO2-ʱ����ҺΪ����Һ��Ȼ���������ӵĹ�������������ע��������ˮ�е���ɫ��
��� �⣺���Ӿ�Ϊ��ɫ���������Ӿ�����ɫ����Һ���ϣ��������������ò���������Ҳ��������ò���������
��1����Һ�����۷�Ӧ����Al3+���ɣ����ӷ�Ӧ����ʽΪ��2Al+6H+=2Al3++3H2������Һ�����ԣ���HCO3-��OH-�����ڣ����������������Ӧһ��û���������������Ҳ������NO3-��������Һ���Ե��ԣ�һ�����������ӣ�����Һ�п϶���Cl-����Ag+����Cl-���ɳ�����˵��ԭ��Һ��Ҳ������Ag+������Һ��һ�����д�����H+��Cl-�����ܺ�Na+��Mg2+��һ�����ܺ��У�Ag+��OH-��HCO3-��NO3-���ʴ�Ϊ��2Al+6H+=2Al3++3H2����H+��Cl-��Ag+��OH-��HCO3-��NO3-��
��2����Һ�����۷�Ӧ����AlO2-���ɣ����ӷ�Ӧ����ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2������Һ�Լ���ʱ��Mg2+��H+��Ag+��HCO3-���ܴ��ڣ�������Һ���Ե��ԣ�һ�����������ӣ���������ֻ��Na+��˵��ԭ��Һ�е�������һ����OH-�����ܺ�NO3-��Cl-���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����Na+��OH-��Mg2+��H+��Ag+��HCO3-��
���� ���⿼�����ʵļ��鼰���ӵĹ������⣬��ȷ��������֮��ķ�Ӧ�ǽ����Ĺؼ�����ע��������ҺΪ�����ԡ���Һ������Ե���������ɣ���Ŀ�ѶȲ���

| A�� | 5�� | B�� | 6�� | C�� | 7�� | D�� | 8�� |
| A�� | 2.3 g����ˮ��Ӧ���������ķ�����Ϊ0.05NA | |
| B�� | 1 mol Fe�������ϡHNO3��Ӧ��ת��2NA������ | |
| C�� | 0.1 mol/LNa2SO4��Һ����0.1NA��SO42- | |
| D�� | 22.4 L����������ͭ��ַ�Ӧ������1 mol CuCl2 |
| A�� | Y�������������ӻ����� | |
| B�� | X���⻯������ˮ������ | |
| C�� | ����ʱZ���ʵ������Ա�X���ʵ��������� | |
| D�� | X�� Z������������Ӧ��ˮ���ﶼ������ |
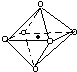
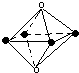
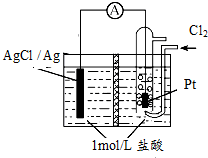 ijԭ���װ����ͼ��ʾ���м���ij�����ӽ���Ĥ������ܷ�ӦΪ2Ag+Cl2�T2AgCl������˵����ȷ���ǣ�������
ijԭ���װ����ͼ��ʾ���м���ij�����ӽ���Ĥ������ܷ�ӦΪ2Ag+Cl2�T2AgCl������˵����ȷ���ǣ�������| A�� | ��طŵ�ʱ�ĸ�����ӦΪAg-e-=Ag+ | |
| B�� | ��ʹ�������ӽ���Ĥ������·��ת��0.01 mol e-ʱ������Ĥ�����Һ��������36.5g | |
| C�� | ��Pt���缫�����˵���֮�⣬���д��������� | |
| D�� | �øõ�ظ�Ǧ���س�磬����11.2L��Cl2�μӵ�ط�Ӧ����������Ǧ���صĸ�����������48g |
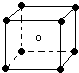
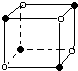
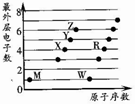 X��Y��Z��M��W��RΪ���ֶ�����Ԫ�أ����ǵ�����������ԭ��������ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
X��Y��Z��M��W��RΪ���ֶ�����Ԫ�أ����ǵ�����������ԭ��������ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | X2M2��W2Z2��Ϊֱ���͵Ĺ��ۻ����� | |
| B�� | X����RZ2��Ӧ����R��XZ��֤��X�ǽ����Ա�Rǿ | |
| C�� | X��Y��Z�ֱ���MԪ���γɵļ�������ȶ������εݼ� | |
| D�� | 1mol��W��MԪ����ɵĻ������������ˮ��ȫ��Ӧ������2g���� |
| A�� | ����Һ����ˮ�������c��H+��=l��10-13mol/L | |
| B�� | 0.1mol•L-1 HA��Һ��0.05mol•L-1 NaOH��Һ�������Ϻ�������Һ�У�c��H+��+c��Na+��=c��OH-��+c��A-�� | |
| C�� | ��pH=4��HA��pH=10��NaOH��Һ�������ϣ���Һ��c��Na+����c��A-����c��OHһ����c��H+�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1mol/L��HA��NaA��Һ�������ϣ�����Һ�����ԣ���c��A-����c��HA����c��Na+����c��H+����c��OH-�� |
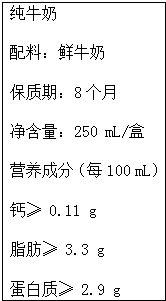 ��Ҷ�֪�� 6��1�� �ǹ��ʶ�ͯ�ڣ���������������˲����˽�����ͬʱҲ�ǡ�����ţ���ա���20����50����������Ĵٽ�ţ������Э���������ף��ţ���ա������룬��������ÿ��5�µ����ܵ��ܶ�Ϊ������ţ���ա��ľ�����2000�꾭���Ϲ���ũ��֯��FAO�������飬��˵�ijЩ�����Ѿ�ȷ�������ڣ�������������700��λ��ҵ����ʿ���������ÿ��� 6��1�� ȷ��Ϊ������ţ���ա��������еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ��塲Ca10��PO4��6��OH��2����ʽ���ڣ�ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺
��Ҷ�֪�� 6��1�� �ǹ��ʶ�ͯ�ڣ���������������˲����˽�����ͬʱҲ�ǡ�����ţ���ա���20����50����������Ĵٽ�ţ������Э���������ף��ţ���ա������룬��������ÿ��5�µ����ܵ��ܶ�Ϊ������ţ���ա��ľ�����2000�꾭���Ϲ���ũ��֯��FAO�������飬��˵�ijЩ�����Ѿ�ȷ�������ڣ�������������700��λ��ҵ����ʿ���������ÿ��� 6��1�� ȷ��Ϊ������ţ���ա��������еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ��塲Ca10��PO4��6��OH��2����ʽ���ڣ�ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺