��Ŀ����
4��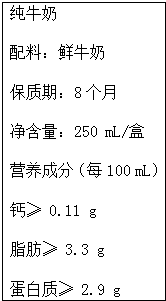 ��Ҷ�֪�� 6��1�� �ǹ��ʶ�ͯ�ڣ���������������˲����˽�����ͬʱҲ�ǡ�����ţ���ա���20����50����������Ĵٽ�ţ������Э���������ף��ţ���ա������룬��������ÿ��5�µ����ܵ��ܶ�Ϊ������ţ���ա��ľ�����2000�꾭���Ϲ���ũ��֯��FAO�������飬��˵�ijЩ�����Ѿ�ȷ�������ڣ�������������700��λ��ҵ����ʿ���������ÿ��� 6��1�� ȷ��Ϊ������ţ���ա��������еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ��塲Ca10��PO4��6��OH��2����ʽ���ڣ�ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺
��Ҷ�֪�� 6��1�� �ǹ��ʶ�ͯ�ڣ���������������˲����˽�����ͬʱҲ�ǡ�����ţ���ա���20����50����������Ĵٽ�ţ������Э���������ף��ţ���ա������룬��������ÿ��5�µ����ܵ��ܶ�Ϊ������ţ���ա��ľ�����2000�꾭���Ϲ���ũ��֯��FAO�������飬��˵�ijЩ�����Ѿ�ȷ�������ڣ�������������700��λ��ҵ����ʿ���������ÿ��� 6��1�� ȷ��Ϊ������ţ���ա��������еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ��塲Ca10��PO4��6��OH��2����ʽ���ڣ�ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺��1���ǻ�������и�Ԫ�ص���������Ϊ39.8%�������� 0.1g ��
��2����װ��ǩ��֬����3.3g����ָ100mLţ���к�֬������ 3.3g����ôһ��ţ���к�������0.28 g���������� 0.01g ��
���� ��1����ѧʽ��ijԪ����������=$\frac{��Ԫ�����ԭ����������Ԫ��ԭ�Ӹ���}{���������ԭ������}$��100%��
��2��100mLţ���к�֬������ 3.3g����CaԪ�غ�������Ϊ0.11g���ݴ˼���һ��ţ���к���������Сֵ��
��� �⣺��1��Ca 10��PO 4�� 6��OH�� 2�и�Ԫ�ص���������Ϊ��$\frac{40��10}{40��10+95��6+17��2}$��100/5=39.8%��
�ʴ�Ϊ��39.8%��
��2��һ��ţ���к�������Ϊ��0.11g��$\frac{250mL}{100mL}$��0.28g���ʴ�Ϊ��0.28g��
���� ���⿼�����������йؼ��㣬�Ƚϻ����������ڻ���֪ʶ�Ĺ��̣�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
15��������Ԫ��W��X��Y��Z��ԭ������������������W�������ӵĺ����������X��Y��Zԭ�ӵĺ����ڲ��������ͬ��X��һ�ֺ����ڿ���ʱ����������һЩ������������ҵ�ϲ���Һ̬��������������Y�ĵ��ʣ�Y���ʻ�ѧ���ʲ����ã���Z���γ�˫ԭ�ӷ��ӣ�������õķǽ������ʣ�������������������˵������ȷ���ǣ�������
| A�� | ��������Ԫ�ص�ԭ�Ӱ뾶��СΪW��X��Y��Z | |
| B�� | ZԪ�ص�����ϼ�Ϊ+7�� | |
| C�� | W��X��Y��Zԭ�ӵĺ����������������ܺ�Ϊ17 | |
| D�� | YԪ�ص����������Ķ�Ӧˮ�������ܽ����еĽ��� |
12��2009��3��22�� �ǵ�17������ˮ�գ�world water day������ˮ������ͨ��ʹ��������������ɱ��������������ˮ��Ӧ�IJ���֮һ�����ᣮ�г�����Щ�����̷�ΪIJȡ����������ˮð�䴿��ˮ������ˮ�����ۣ�Ϊ�����α����������һ�ֻ�ѧ�Լ����𣬸��Լ��ǣ�������
| A�� | ��̪��Һ | B�� | �Ȼ�����Һ | C�� | ����������Һ | D�� | ��������Һ |
19���ò������ֱ�պȡ���и����е�����Ũ��Һ�������������¶��������û�а������ɵ��ǣ�������
| A�� | Ũ��ˮ��ŨHNO3 | B�� | Ũ��ˮ��Ũ���� | C�� | Ũ��ˮ��ŨH2SO4 | D�� | Ũ��ˮ��ŨH3PO4 |
16�����з�Ӧ�У����ڼӳɷ�Ӧ���ǣ�������
| A�� | CH3COOCH2CH3+H2O $\stackrel{������}{��}$ CH3CH2OH+CH3COOH | |
| B�� | CH2=CH2+HCl$��_{��}^{һ������}$CH3CH2Cl | |
| C�� | 2CH3CH2OH+O2$\stackrel{Cu}{��}$2CH3CHO+2H2O | |
| D�� |  |
14�� ��1����֪Ԫ��M���������NH4Al��SO4��2��һ��Ԫ�أ�Ԫ��M����̬ԭ�����ʧȥ��1������4�����������������������ܣ��÷���I1��I4��ʾ�������ʾ��
��1����֪Ԫ��M���������NH4Al��SO4��2��һ��Ԫ�أ�Ԫ��M����̬ԭ�����ʧȥ��1������4�����������������������ܣ��÷���I1��I4��ʾ�������ʾ��
Ԫ��M������������
��2����ͬԪ�ص�ԭ���ڷ������������ӵ�������С���õ縺������ʾ��������ijЩ������Ԫ�ص縺�ԣ�
��ͨ�������縺�Ա仯���ɣ�ȷ��Mg�ĵ縺�ԣ���x��ʾ����Сȡֵ��Χ��0.9��x��1.57��
��ij�л�������ṹʽΪ ������S-N�У�����Ϊ���õ��Ӷ�ƫ��˭������дԭ�����ƣ�
������S-N�У�����Ϊ���õ��Ӷ�ƫ��˭������дԭ�����ƣ�
����֪Ԫ�ص縺�ԵIJ�ֵһ�����1.7ʱ��ԭ�Ӽ��γ����Ӽ���С�ڸ�ֵ��ԭ�Ӽ��γɹ��ۼ�����ָ������ԭ��֮���γɵĻ�ѧ�������Ӽ����ǹ��ۼ���Be��F���Ӽ���Si��Cl���ۼ���
��3����ҵ��ұ��Zʱ�õ��Ĵ����DZ���ʯ����ȡ����ʯ��Na3AlF6���Ļ�ѧ����ʽ���£�
2Al��OH��3+12HF+3A�T2Na3AlF6+3CO2��+9H2O
�ٷ�Ӧ��A�Ļ�ѧʽΪ Na2CO3���������Ӿ���
�ڱ���ʯ��Na3AlF6�������ӻ���������������ɣ�����ʯ�����ṹ��ͼ��ʾ������λ�ڴ������嶥������ģ�����λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ�������������������Na+�������������ţ�
����Na3AlF6��Ħ������ΪMg/mol�����þ����ı߳�Ϊacm���ܶ�Ϊ��g/cm3����NA��ʾ����٤����������NA=$\frac{4M}{��{a}^{3}}$/mol����M��a���ѱ�ʾ����
 ��1����֪Ԫ��M���������NH4Al��SO4��2��һ��Ԫ�أ�Ԫ��M����̬ԭ�����ʧȥ��1������4�����������������������ܣ��÷���I1��I4��ʾ�������ʾ��
��1����֪Ԫ��M���������NH4Al��SO4��2��һ��Ԫ�أ�Ԫ��M����̬ԭ�����ʧȥ��1������4�����������������������ܣ��÷���I1��I4��ʾ�������ʾ��| I1 | I2 | I3 | I4 | |
| �����ܣ�kJ/mol�� | 578 | 1817 | 2745 | 11578 |
��2����ͬԪ�ص�ԭ���ڷ������������ӵ�������С���õ縺������ʾ��������ijЩ������Ԫ�ص縺�ԣ�
| Ԫ�ط��� | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
| �縺�� | 1.0 | 1.57 | 2.0 | 2.5 | 3.5 | 4.0 | 0.9 | 1.61 | 1.8 | 2.1 | 2.5 | 3.0 |
��ij�л�������ṹʽΪ
 ������S-N�У�����Ϊ���õ��Ӷ�ƫ��˭������дԭ�����ƣ�
������S-N�У�����Ϊ���õ��Ӷ�ƫ��˭������дԭ�����ƣ�����֪Ԫ�ص縺�ԵIJ�ֵһ�����1.7ʱ��ԭ�Ӽ��γ����Ӽ���С�ڸ�ֵ��ԭ�Ӽ��γɹ��ۼ�����ָ������ԭ��֮���γɵĻ�ѧ�������Ӽ����ǹ��ۼ���Be��F���Ӽ���Si��Cl���ۼ���
��3����ҵ��ұ��Zʱ�õ��Ĵ����DZ���ʯ����ȡ����ʯ��Na3AlF6���Ļ�ѧ����ʽ���£�
2Al��OH��3+12HF+3A�T2Na3AlF6+3CO2��+9H2O
�ٷ�Ӧ��A�Ļ�ѧʽΪ Na2CO3���������Ӿ���
�ڱ���ʯ��Na3AlF6�������ӻ���������������ɣ�����ʯ�����ṹ��ͼ��ʾ������λ�ڴ������嶥������ģ�����λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ�������������������Na+�������������ţ�
����Na3AlF6��Ħ������ΪMg/mol�����þ����ı߳�Ϊacm���ܶ�Ϊ��g/cm3����NA��ʾ����٤����������NA=$\frac{4M}{��{a}^{3}}$/mol����M��a���ѱ�ʾ����
 ��1��CH3+��-CH3��������CH3-������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ����CDE��
��1��CH3+��-CH3��������CH3-������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ����CDE��