��Ŀ����
����Ŀ��������(SO2Cl2)��ҽҩ��Ⱦ����ҵ����Ҫ��;��Ҳ�������Ʊ�������Լ����е�Ϊ69.2�棬��ˮˮ�⣬���ҷ�Ӧ��������ǿ�ᡣѧϰС����ʵ������SO2��Cl2�ڻ���̿�����£��Ʊ�SO2Cl2���ⶨ��Ʒ���ȣ������ͼʵ����ͼ1(�г�װ����ȥ)����ش��������⣺
��.SO2���Ʊ�
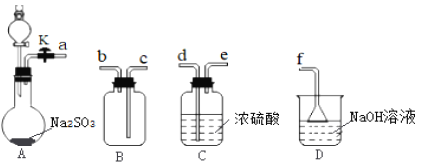
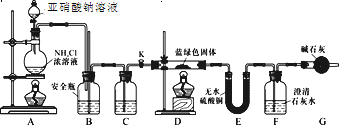
��1�����ռ�һƿ�����SO2��װ���������������������˳��Ϊ____(��Сд��ĸ)��
��2��Aװ�÷�Һ©����װ����70%��������Һ��A�з�Ӧ�Ļ�ѧ����ʽΪ____��
��.SO2Cl2���Ʊ��ʹ��ȵIJⶨ
�������ռ�����SO2����ע����h�У���ͼ2װ���Ʊ�SO2Cl2��

��3������e������Ϊ____��b���Լ�������Ϊ____��
��4��f��������____��
��5��ȡ��Ӧ��IJ�Ʒ4.0g�����200mL��Һ��ȡ��20.00mL����0.5000mol ��L-1NaOH��Һ�ζ����ﵽ�ζ��յ�ʱ���ı�Һ�����Ϊ20.00mL(���ʲ��μӷ�Ӧ)��
�ٲ�Ʒ��ˮ�����Һʱ�����ķ�ӦΪ____��
��SO2Cl2����������Ϊ____��(������λ��Ч����)��
���𰸡�adecbf��adecbdef Na2SO3+H2SO4=Na2SO4+SO2��+H2O �����ܻ����������� ����ʳ��ˮ ��ֹˮ��������dʹSO2Cl2ˮ�� SO2Cl2+2H2O= H2SO4+2HCl 84.4%
��������
(1)�ռ�һƿ�����SO2����������ȡ���壬Ȼ���ᴿ�����ռ�����Ϊ���������ж���������ˮ����Ҫ��β���������մ������ݴ˷������
(2)A�������ɶ�������ķ�Ӧ��
(3)�������ӷ������ɵ������л�����Ȼ��⣬�ݴ˷������
(4)��ˮ�Ȼ��ƾ�����ˮ�ԣ����SO2Cl2��ˮ��ˮ��������
(5)��SO2Cl2��ˮˮ�⣬���ҷ�Ӧ��������ǿ�ᣬ���������������ڸ���NaOH �����ᡢ���ᷴӦ���õ�SO2Cl2�����ʵ������Ӷ��ó�����������
(1)����ͼʾ��װ��A����������ȡ�����������ɵĶ��������л���ˮ����������ͨ��C�и����B�ռ�������Ķ�������������������Һ���գ���ֹ��Ⱦ��������ռ�һƿ�����SO2��װ�õĵ��ܰ������������ӵ�˳���ǣ�aͨ������d�Ѷ����������Լ�ƿ�н��и��ͨ��e���ܵ�����ͨ��c���ܵ��뼯��ƿ���ռ���Ϊ�˷�ֹ���������ݳ���ɢ����������Ⱦ������Ӧ�ð�b�������ӵ�f�����ϣ�Ϊ��ֹͨ��D�����ݳ��Ķ��������ˮ�ݵ�B�У���b�������ӵ�d�Ѷ����������Լ�ƿ�н��и��ͨ��e���ܵ���������f���ʴ�Ϊ��adecbf��adecbdef��
(2)Aװ�÷�Һ©����װ����70%��������Һ�����ɶ�������ķ�Ӧ����ʽΪH2SO4+Na2SO3=SO2��+Na2SO4+H2O���ʴ�Ϊ��H2SO4+Na2SO3=SO2��+Na2SO4+H2O��
(3)����ͼʾ��eΪ���������ܣ�b�������dz�ȥ�����л��е��Ȼ������壬��b���Լ�Ϊ����ʳ��ˮ���ʴ�Ϊ��(����)�����ܣ�����ʳ��ˮ��
(4)SO2Cl2��ˮ��ˮ�⣬��f�����õķ�ֹ�����е�ˮ�������뵽d���ʴ�Ϊ����ֹ�����е�ˮ�������뵽dʹSO2Cl2ˮ�⣻
(5)��SO2Cl2��ˮˮ�⣬���ҷ�Ӧ��������ǿ�ᣬ��Ϊͬʱ������������ᣬ����ʽΪ��2H2O+SO2Cl2=H2SO4+2HCl���ʴ�Ϊ��2H2O+SO2Cl2=H2SO4+2HCl��
�ڸ���2H2O+SO2Cl2=H2SO4+2HCl�õ���SO2Cl2��4H+��4OH-�Ĺ�ϵ�������������Ƶ����ʵ���Ϊ0.5000molL-1��20.00��10-3L=0.01mol����n(SO2Cl2)=![]() =0.0025mol����� 200mL ��Һ��ȡ�� 20.00mL������n(SO2Cl2)��=0.0025mol��10=0.025mol��m(SO2Cl2)=n��M=0.025mol��135g/mol=3.375g����������=
=0.0025mol����� 200mL ��Һ��ȡ�� 20.00mL������n(SO2Cl2)��=0.0025mol��10=0.025mol��m(SO2Cl2)=n��M=0.025mol��135g/mol=3.375g����������=![]() ��100%=84.4%���ʴ�Ϊ��84.4%��
��100%=84.4%���ʴ�Ϊ��84.4%��