��Ŀ����
����Ŀ���ۺ���������һ������Ч�����߷������������������������弰����Ϊԭ�ϴ���������������������ˮ�⡢�ۺϳɲ�Ʒ��ʵ����ģ�������������£�
![]()
��֪Fe3����ˮ�������������Fe3����3H2O===Fe��OH��3��3H����Ϊ�˷�ֹFe3��ˮ����������ᡣ
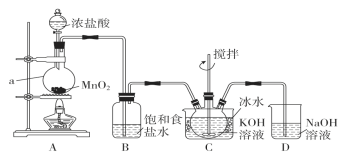
��1������ԭ������2.50 moL��L��1������������Һʱ�õ��Ķ���������_________________��
��д�����������е����ӷ���ʽ��________��
��2���ۺϿ���ʵ��Ͷ��������������������ʵ���֮��Ϊ1/1.25������ѣ���������������ֵ�Զ࣬�����ܹ����ԭ����__________________________________��
��3����������Һˮ����Եõ�һϵ�о��о�ˮ���õļ�ʽ��������xFe2O3��ySO3��zH2O�����ֲ����������ⶨx��y��z��ֵ��
�ٲⶨʱ������Լ���______����ѡ����ţ���
A��NaOH B. Ba��OH��2
C��BaCl2D��FeSO4
����Ҫ�ⶨ________��__________����������д������Ļ�ѧʽ����
��4��ѡ���ⶨ����������Ļ����������������Ⱥ�˳���г��� ______________������ţ���
�ٹ��ˡ�ϴ�ӡ����������ᾧ������ȡ����Һ�� ����ȴ���������ݺ�ɻ�����
���𰸡�������ƽ������ƿ 4Fe2����O2��4H��===4Fe3����2H2O �������ʱ�������к���Ҫ�ļ������������˷� AC Fe2O3 BaSO4 �٢ݢ�
��������
��1��������һ�����ʵ���Ũ�ȵ���ҺҪ�������ʵ�������������ƿ���ݣ�
���������������������±�������������������
��2��������࣬�ں������pHʱҪ���ĵ��������Ƶ����Ͷ࣬����˷ѣ�
��3���ٲ����������ⶨ��ʽ��������xFe2O3ySO3zH2O����x��y��z��ֵʱ�����Խ���Ʒ��������������Һ�����ݵõ��Ĺ���������������ȷ��x��ֵ�����ˣ���������Һ�м��Ȼ��������ݲ��������ᱵ������������ȷ��y��ֵ��������Ʒ�����������Fe2O3�ͼ���õ�SO3��������ȷ��z��ֵ��
�ڸ��ݢٵķ�����֪��Ҫ�ⶨFe2O3��BaSO4��������
��4���ⶨ�����н���Ʒ��������������Һ���������ˡ�ϴ�ӡ���ɻ����ա���ȴ������������������������������Һ�м��Ȼ������������ˡ�ϴ�ӡ���ɻ����ա���ȴ�����������ᱵ���������ݴ˴��⡣
��1��������һ�����ʵ���Ũ�ȵ���ҺҪ�������ʵ�������Ҫ�õ�����ƽ��������ƿ���ݣ�
�𰸣�������ƽ������ƿ��
���������������������±����������������������ӷ���ʽΪ4Fe2++O2+4H+=4Fe3++2H2O��
�𰸣�4Fe2++O2+4H+=4Fe3++2H2O��
��2�������Թ�����Ϊ�˷�ֹFe3+ˮ����������������������ʱ�������к���Ҫ�ļ��������������˷ѣ����Լ�������������ֵ�Զ࣬�����ܹ��ࣻ
�𰸣��������ʱ�������к���Ҫ�ļ������������˷� ��
��3���ٲ����������ⶨ��ʽ��������xFe2O3ySO3zH2O����x��y��z��ֵʱ�����Խ���Ʒ��������������Һ�����ݵõ��Ĺ���������������ȷ��x��ֵ�����ˣ���������Һ�м��Ȼ��������ݲ��������ᱵ������������ȷ��y��ֵ��������Ʒ�����������Fe2O3�ͼ���õ�SO3��������ȷ��z��ֵ��
�𰸣�AC��
�ڸ��ݢٵķ�����֪��Ҫ�ⶨFe2O3��BaSO4��������
�𰸣�Fe2O3��BaSO4��
��4���ⶨ�����н���Ʒ��������������Һ���������ˡ�ϴ�ӡ���ɻ����ա���ȴ������������������������������Һ�м��Ȼ������������ˡ�ϴ�ӡ���ɻ����ա���ȴ�����������ᱵ��������
�𰸣� �٢ݢܡ�

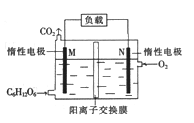
����Ŀ����ͼ��ʾװ����,�ס��ҡ��������ձ����ηֱ�ʢ��������NaCl��Һ��AgNO3��Һ��x��Һ,a��b��c��d�缫��Ϊʯī�缫![]() ��ͨ��Դ,����һ��ʱ���,����c�缫��������
��ͨ��Դ,����һ��ʱ���,����c�缫��������![]() �ݴ˻ش����⣺
�ݴ˻ش����⣺
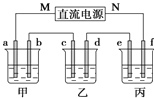
(1)��Դ��M��Ϊ_____________����
(2)�缫d�Ϸ����ĵ缫��ӦʽΪ__________�� �ҳ���ҺPH__________���������������С��������������
(3)�׳��е��ܷ�ӦʽΪ___________________________________��
(4)����·����0.04mol����ͨ��ʱ,a��b��c��d�缫�ϲ�����������������ʵ���֮����____________��
(5)�����ñ���ʵ�����϶�ͭ,����e-f-x��![]() ��Һ��__________________________��(Ҫ��e��f��x�þ������ʻش�,��ͬ),�����ñ���ʵ�ֵ�⾫��ͭ,��f�缫������_______________________
��Һ��__________________________��(Ҫ��e��f��x�þ������ʻش�,��ͬ),�����ñ���ʵ�ֵ�⾫��ͭ,��f�缫������_______________________
(6) ʵ����, 1g�״���CH3OH��Һ���������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�22.68kJ������,���ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��_____________________________________________________
(7)��������![]() �ṹʽΪ
�ṹʽΪ![]() ���л��ϳ��е���Ҫ�Լ�,����
���л��ϳ��е���Ҫ�Լ�,����![]() ��
��![]() ��ͨ����Ӧ�������Ƶ�,��Ӧ����ʽΪ
��ͨ����Ӧ�������Ƶ�,��Ӧ����ʽΪ![]() ����֪���ֻ�ѧ���ļ����������±���ʾ��
����֪���ֻ�ѧ���ļ����������±���ʾ��
��ѧ�� |
|
|
|
|
���� | 243 | a | 607 | 630 |
��![]() ��NO��Ӧ����ClNO�Ĺ�����ת����4mol����,�����Ϸų�������Ϊ______kJ��(�����ֺ���ĸ��ʾ)
��NO��Ӧ����ClNO�Ĺ�����ת����4mol����,�����Ϸų�������Ϊ______kJ��(�����ֺ���ĸ��ʾ)