��Ŀ����
�о���ѧ��Ӧ�е������仯����Ҫ���塣�����ѧ��֪ʶ�ش��������⣺
��1����֪һ����̼��ˮ������Ӧ���̵������仯����ͼ��ʾ��

�ٷ�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
����֪��

��

��2����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γɣ����1 mol��ѧ��ʱ�ͷţ������գ�����������֪��N��N���ļ�����948.9kJ��mol��1��H��H���ļ�����436.0 kJ��mol��1�� N��H���ļ�����391.55 kJ��mol��1����1/2N2(g) + 3/2H2(g) ="=" NH3(g) ��H = ��
��1����֪һ����̼��ˮ������Ӧ���̵������仯����ͼ��ʾ��

�ٷ�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
����֪��


��


��2����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γɣ����1 mol��ѧ��ʱ�ͷţ������գ�����������֪��N��N���ļ�����948.9kJ��mol��1��H��H���ļ�����436.0 kJ��mol��1�� N��H���ļ�����391.55 kJ��mol��1����1/2N2(g) + 3/2H2(g) ="=" NH3(g) ��H = ��
��1����CO(g)+H2O(g)=CO2(g)+H2(g) ��H=��41KJ/mol�� �ڡ�H=+172KJ/mol��
��2����46.2 KJ/moL
��2����46.2 KJ/moL
�����������1���������仯ͼʾ��֪�÷�Ӧ���Ȼ�ѧ����ʽΪ����CO(g)+H2O(g)=CO2(g)+H2(g) ��H=��41KJ/mol���ڽ�CO(g)+H2O(g)=CO2(g)+H2(g)��ȥC(s)+H2O(g)=CO(g)+H2(g)�������ɵã�C(s)+CO2(g)-2CO(g) ��H=+172KJ/mol����2�����ݼ��ܵĶ��弰�뷴Ӧ�ȵĹ�ϵ��֪�� ��H="1/2��948.9kJ/mol+3/2��436.0" kJ/mol-3��391.55kJ/mol="��46.2" KJ/moL

��ϰ��ϵ�д�
 �������ϵ�д�
�������ϵ�д�
�����Ŀ
 CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
 ��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��
��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��
 H2(g)��
H2(g)�� kJ��mol��1
kJ��mol��1
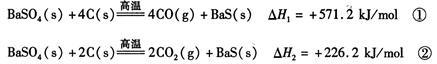
 2CO(g)�ġ�H = kJ/mol
2CO(g)�ġ�H = kJ/mol = ��[��֪��
= ��[��֪�� CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0
 CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��
CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��

 CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1�������
CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1�������