��Ŀ����
��15�֣���Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO(NH2)2]����֪��
��2NH3��g���� CO2��g���� NH2CO2NH4��s�� ��H �� -159.47 kJ��mol-1
��NH2CO2NH4��s���� CO(NH2)2��s���� H2O��g�� ��H �� +116.49 kJ��mol-1
��H2O��l���� H2O��g�� ��H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ ��
��2����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�
CO2(g)+4H2(g) CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0
����һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L��1��H2��0.8mol��L��1��CH4��0.8mol��L��1��H2O��1.6mol��L��1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ �� ��CO2��ƽ��ת����Ϊ ��
������������ͬ�ĺ��ݾ��ȣ������û�������������ܱ�����I��II����I�г���1 molCO2,��4 molH2����II�г���1 mol CH4��2 mol H2 O(g) ��300���¿�ʼ��Ӧ���ﵽƽ��ʱ������˵����ȷ���� ������ĸ����
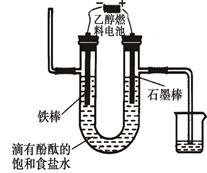
��3����ʢ�ٴ�ѧ���о���Ա�о���һ�ַ�������ʵ��ˮ������ʱCO2���ŷţ������ԭ����ͼ��ʾ��
�������������̵�����ת����ʽ�� ��
��������ⷴӦ���¶�С��900��ʱ����̼����ȷֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ �������ĵ缫��ӦʽΪ ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO(NH2)2]����֪��
��2NH3��g���� CO2��g���� NH2CO2NH4��s�� ��H �� -159.47 kJ��mol-1
��NH2CO2NH4��s���� CO(NH2)2��s���� H2O��g�� ��H �� +116.49 kJ��mol-1
��H2O��l���� H2O��g�� ��H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ ��
��2����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�
CO2(g)+4H2(g)
 CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0����һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L��1��H2��0.8mol��L��1��CH4��0.8mol��L��1��H2O��1.6mol��L��1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ �� ��CO2��ƽ��ת����Ϊ ��
������������ͬ�ĺ��ݾ��ȣ������û�������������ܱ�����I��II����I�г���1 molCO2,��4 molH2����II�г���1 mol CH4��2 mol H2 O(g) ��300���¿�ʼ��Ӧ���ﵽƽ��ʱ������˵����ȷ���� ������ĸ����
| A������I��II������Ӧ������ͬ |
| B������I��II��CH4�����ʵ���������ͬ |
| C������I��CO2�����ʵ���������II�еĶ� |
| D������I��CO2��ת����������II��CH4��ת����֮��С��1 |

�������������̵�����ת����ʽ�� ��
��������ⷴӦ���¶�С��900��ʱ����̼����ȷֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ �������ĵ缫��ӦʽΪ ��
��1��2NH3��g���� CO2��g����CO(NH2)2��s����H2O��l����H ����130.98 kJ��mol��1 ��3�֣�
��2����2mol��1�֣���8mol��1�֣���80% ��2�֣� ��CD��2�֣�
��3����̫���ܺ͵���ת��Ϊ��ѧ�ܣ�2�֣�
��2CO32����4e��=2CO2��+O2����2�֣���3CO2+4e��=C+2CO32����2�֣�
��2����2mol��1�֣���8mol��1�֣���80% ��2�֣� ��CD��2�֣�
��3����̫���ܺ͵���ת��Ϊ��ѧ�ܣ�2�֣�
��2CO32����4e��=2CO2��+O2����2�֣���3CO2+4e��=C+2CO32����2�֣�
�����������1���ɢ٣��ڣ��ۿɵã�2NH3(g)�� CO2(g)��CO(NH2)2(s)��H2O(l) ��H ����130.98 kJ��mol��1 ��3�֣�
��2���ټ�����ʼ�Ķ�����̼�����ʵ���Ϊxmol�����������ʵ���Ϊymol��
CO2(g) + 4H2(g)
 CH4(g) + 2H2O(g) ��H��0
CH4(g) + 2H2O(g) ��H��0��ʼ���ʵ����� xmol ymol 0 0
ת�����ʵ����� 1.6mol 6.4mol 1.6mol 3.2mol
ƽ�����ʵ����� 0.4mol 1.6mol 1.6mol 3.2mol
��ô��x��0.4mol��1.6mol��2.0mol y��6.4mol��1.6mol��8.0mol
CO2��ƽ��ת����Ϊ��
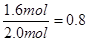
��A������I��II�е���ʼ��Ӧ�ﲻһ�����������Ӧ���ʲ�һ����ȣ�����B���������һ��������������ô�����������а����е�Ͷ���ǵ�Чƽ�⣬Ҳ����˵���������м���ĺ���Ӧ����ȣ�������һ�����ȵ�����������Ƕ�����̼���������ɼ����ˮ������Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ�����¶����ߣ������¶Ȼ�ʹ��ƽ�������ƶ���ʹ�ü���ĺ�����ǰ���ĵ�Чƽ��ʱ��Ҫ�ͣ���Ͷ������ˮ�����ɵ��Ƕ�����̼��������Ҫ���ȣ�ʹ����ϵ���¶Ƚ��ͣ����¶ȵĽ���ʹ��ƽ�������ƶ���ʹ�ü���ĺ�����ǰ���ĵ�Чƽ��ʱ��Ҫ�ߣ��������I��II��CH4�����ʵ�����������ͬ������C������I������ǰ����Чƽ��Ļ����������ƶ�������II������ǰ��ʶ����Чƽ��Ļ����������ƶ�����������I��CO2�����ʵ���������II�еĶ࣬��ȷ��D��������յ�Чƽ�������ǵĻ�������I��CO2��ת����������II��CH4��ת����֮�͵���1���������ھ��������У����ڵ�Чƽ��Ļ������ֱַ�������ƶ�����ˣ�����I��CO2��ת����������II��CH4��ת����֮��С��1����ȷ��
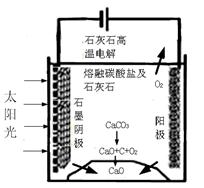
��3���ٴ�ͼ�п�֪װ���ǽ�̫���ܺ͵���ת��Ϊ��ѧ�ܣ�2�֣�
��������ʧȥ���ӵķ�Ӧ��2CO32����4e��=2CO2��+O2���������ǵõ����ӵķ�Ӧ���õ��ӵ�����ֻ���Ƕ�����̼��3CO2+4e��=C+2CO32����

��ϰ��ϵ�д�
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
�����Ŀ
 CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ� N2��2CO2����÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��________��
N2��2CO2����÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��________��
 2N2(g)��3H2O(g)����H<0���ں��ݵ��ܱ������У������й�˵����ȷ����________��
2N2(g)��3H2O(g)����H<0���ں��ݵ��ܱ������У������й�˵����ȷ����________��





 2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol
2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol