��Ŀ����
����Ŀ���ϳ�������Ҫ�ɷ���һ����̼�������������ںϳɶ����ѵ����ȼ�ϡ�����Ȼ����úϳ��������п��ܷ����ķ�Ӧ�У�
CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+206.1kJ/mol ��
CO(g)+3H2(g) ��H1=+206.1kJ/mol ��
CH4(g)+CO2(g)![]() 2CO(g)+2H2(g) ��H2=+247.3kJ/mol ��
2CO(g)+2H2(g) ��H2=+247.3kJ/mol ��
��ش��������⣺
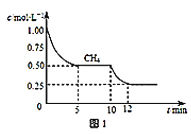
(1)��һ�ܱ������н��з�Ӧ�٣����CH4�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ����Ӧ���е�ǰ5min�ڣ�v(H2)=____��10minʱ���ı���������������_____��
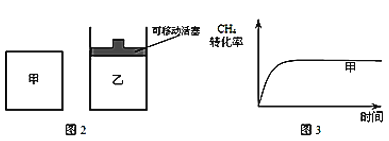
(2)��ͼ2��ʾ���ڼס����������зֱ��������ʵ�����CH4��CO2��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ�ڣ���ά�ַ�Ӧ�������¶Ȳ��䡣��֪��������CH4��ת������ʱ��仯��ͼ����ͼ3��ʾ������ͼ3�л�����������CH4��ת������ʱ��仯��ͼ��_____��
(3)800��ʱ����ӦCO(g)+H2O(g)![]() CO2(g)+H2(g)�Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±�����ʱ��Ӧ�������淴Ӧ���ʵĹ�ϵʽ��____������ţ���
CO2(g)+H2(g)�Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±�����ʱ��Ӧ�������淴Ӧ���ʵĹ�ϵʽ��____������ţ���
a.v(��)��v(��)
b.v(��)��v(��)
c.v(��)=v(��)
d.���ж�
CO | H2O | CO2 | H2 |
0.5mol | 8.5mol | 2.0mol | 2.0mol |
(4)�����ѣ�CH3OCH3�����ɺϳ�����CO��H2����һ���������Ƶã�д���÷�Ӧ�Ļ�ѧ����ʽ��____��
(5)�Զ����ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫����ȼ�ϵ�ء��õ�صĸ����缫��Ӧ����ʽ�ǣ�____���ö�����ȼ�ϵ��Ϊ��Դ���ò��缫���KCl��CuSO4�Ļ����Һ������·��ͨ��0.3mol���ӵĵ���ʱ����������������2.8L������(��״��)����������Һ���Ϊ1L������������ijɷּ����ʵ���Ϊ��____��
���𰸡�0.3mol/(L��min) �����¶Ȼ����ˮ����  a 2CO+4H2��CH3OCH3+H2O��һ�������� CH3OCH3+16OH--12e-=2CO32-+11H2O O2��0.025mol��Cl2��0.10mol
a 2CO+4H2��CH3OCH3+H2O��һ�������� CH3OCH3+16OH--12e-=2CO32-+11H2O O2��0.025mol��Cl2��0.10mol
��������
(1)��Ӧ���е�ǰ5min�ڣ�CH4��Ũ�ȱ仯��Ϊ0.50mol/L���ɴ˿����v(H2)��10minʱ���ı�����������2min��CH4��Ũ�ȱ仯��Ϊ0.25mol/L����ǰһʱ��ν��жԱȣ��Ӷ��ó���Ӧ���ʼӿ죬�ɴ�Ԥ����ܷ����ı�����������
(2)��Ϊ��ӦCH4(g)+CO2(g)![]() 2CO(g)+2H2(g)������������������������������൱�ڼ�������Сѹǿ���ɴ˿�ȷ��ƽ���ƶ��ķ���CH4��ת���ʱ仯�������ʱ�䡣
2CO(g)+2H2(g)������������������������������൱�ڼ�������Сѹǿ���ɴ˿�ȷ��ƽ���ƶ��ķ���CH4��ת���ʱ仯�������ʱ�䡣
(3)800��ʱ����ӦCO(g)+H2O(g)![]() CO2(g)+H2(g)�Ļ�ѧƽ�ⳣ��K=1.0�����ñ���������Ũ���̣�����K���бȽϣ��Ӷ�ȷ��ƽ���ƶ��ķ����ɴ˵ó������淴Ӧ���ʵ���Դ�С��
CO2(g)+H2(g)�Ļ�ѧƽ�ⳣ��K=1.0�����ñ���������Ũ���̣�����K���бȽϣ��Ӷ�ȷ��ƽ���ƶ��ķ����ɴ˵ó������淴Ӧ���ʵ���Դ�С��
(4) CO��H2��һ���������Ƶ�CH3OCH3���ɴ˿�д���÷�Ӧ�Ļ�ѧ����ʽ��
(5)�Զ����ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫����ȼ�ϵ�ء��õ�صĸ���Ϊ�������ڼ���������ʧ���ӣ�ת��ΪCO32-�ȡ��ö�����ȼ�ϵ��Ϊ��Դ���ò��缫���KCl��CuSO4�Ļ����Һ�������ȷ���Cl-ʧ��������Cl2�ķ�Ӧ������H2Oʧ��������O2�ķ�Ӧ�������ȷ���Cu2+�õ��ӵķ�Ӧ������H2O�õ�������H2�ķ�Ӧ���������ݽ�������ʽ��������������
(1)��ͼ�п���ȡ������Ϣ����Ӧ���е�ǰ5min�ڣ�CH4��Ũ�ȱ仯��Ϊ0.50mol/L��10min�ı�ij������2min��CH4��Ũ�ȱ仯��Ϊ0.25mol/L����Ӧ���е�ǰ5min�ڣ�v(CH4)=![]() =0.10mol/(L��min)�����ݻ�ѧ����ʽCH4(g)+H2O(g)
=0.10mol/(L��min)�����ݻ�ѧ����ʽCH4(g)+H2O(g)![]() CO(g)+3H2(g)���ɵó�v(H2)=3v(CH4)= 0.30mol/(L��min)����10minǰ���жԱȣ���Ӧ���ʼӿ���ƽ�������ƶ������Ըı��������������������¶Ȼ����ˮ��������Ϊ��0.30mol/(L��min)�������¶Ȼ����ˮ������
CO(g)+3H2(g)���ɵó�v(H2)=3v(CH4)= 0.30mol/(L��min)����10minǰ���жԱȣ���Ӧ���ʼӿ���ƽ�������ƶ������Ըı��������������������¶Ȼ����ˮ��������Ϊ��0.30mol/(L��min)�������¶Ȼ����ˮ������
(2)�����Լ�Ϊ���գ����ŷ�Ӧ�Ľ��У��������������ѹǿ�����ں�ѹ�����ң��൱�ڼ������ݻ���Сѹǿ����Ӧ���ʼ�������ƽ�������ƶ���CH4��ת���������ɴ˿���ͼ3�л�����������CH4��ת������ʱ��仯��ͼ��Ϊ ����Ϊ��
������ ��
��
(3)�ɱ������ݼ���ӦCO(g)+H2O(g)![]() CO2(g)+H2(g)�������Ũ����ΪQ=
CO2(g)+H2(g)�������Ũ����ΪQ=![]() =0.94<K������ƽ�������ƶ���v(��)��v(��)����ѡa����Ϊ��a��
=0.94<K������ƽ�������ƶ���v(��)��v(��)����ѡa����Ϊ��a��
(4) CO��H2��һ���������Ƶ�CH3OCH3����ѧ����ʽΪ2CO+4H2![]() CH3OCH3+H2O����Ϊ��2CO+4H2
CH3OCH3+H2O������2CO+4H2![]() CH3OCH3+H2O��
CH3OCH3+H2O��
(5)�Զ����ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫����ȼ�ϵ�ء��õ�صĸ���Ϊ�������ڼ���������ʧ���ӣ�ת��ΪCO32-�ȣ��缫��Ӧ����ʽ�ǣ�CH3OCH3+16OH--12e-=2CO32-+11H2O��
���������ķ�ӦΪ��2Cl--2e-span>==Cl2����2H2O-4e-==O2��+4H+��n(����)=![]() =0.125mol����n(Cl2)=x����n(O2)=0.125-x���Ӷ��ó�2x+4��(0.125-x)=0.3mol���Ӷ����x=0.10mol��0.125-x=0.025mol����O2��0.025mol��Cl2��0.10mol����Ϊ��O2��0.025mol��Cl2��0.10mol��
=0.125mol����n(Cl2)=x����n(O2)=0.125-x���Ӷ��ó�2x+4��(0.125-x)=0.3mol���Ӷ����x=0.10mol��0.125-x=0.025mol����O2��0.025mol��Cl2��0.10mol����Ϊ��O2��0.025mol��Cl2��0.10mol��

����Ŀ����1L��0.01molNaAlO2��![]() ����Һ�л���ͨ�������̼����
����Һ�л���ͨ�������̼����![]() �����Ⱥ���������ͬ�ķ�Ӧ����0.01mol<n(CO2)��0.015molʱ�����ķ�Ӧ��:2NaAlO2+CO2+3H2O=2Al(OH)3��+Na2CO3�����ж�Ӧ��ϵ��ȷ���ǣ� ��
�����Ⱥ���������ͬ�ķ�Ӧ����0.01mol<n(CO2)��0.015molʱ�����ķ�Ӧ��:2NaAlO2+CO2+3H2O=2Al(OH)3��+Na2CO3�����ж�Ӧ��ϵ��ȷ���ǣ� ��
ѡ�� |
| ��Һ�����ӵ����ʵ���Ũ�� |
A | 0 |
|
B | 0.01 |
|
C | 0.015 |
|
D | 0.03 |
|
A.AB.BC.CD.D
����Ŀ������ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��������Fe2O3��A12O3��MnO��MgO��CaO��)��ȡ�����ܵĹ���������ͼ��
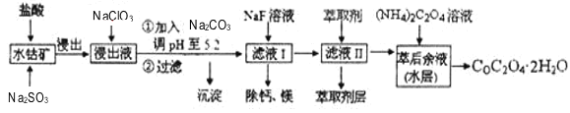
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Al(OH)3 | Co(OH)2 | Fe(OH)2 | Mn(OH)2 |
��ȫ������pH | 3.7 | 5.2 | 9.2 | 9.6 | 9.8 |
(1)���������м���Na2SO3��Ŀ����_____��
(2)NaClO3�ڷ�Ӧ����Ԫ�ر���ԭΪ��ͼۣ��÷�Ӧ�����ӷ���ʽΪ_____��
(3)��Na2CO3��ʹ����Һ��ijЩ��������ת������������������������ӷ���ʽ�ͱ�Ҫ�����ּ�����ԭ����_____��
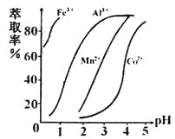
(4)��ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ��ʾ������ҺII��������ȡ��pHΪ_____���ҡ�
a.2.0~2.5 b.3.0~3.5 c.4.0~4.5
(5)��ҺI�����ơ�þ���ǽ���ת��ΪMgF2��CaF2��������֪Ksp(MgF2)=7.35��10-11��Ksp(CaF2)=1.05��10-10�����������NaF��������Һ![]() =____��
=____��
(6)��ҵ���ð�ˮ���շ����е�SO2����֪NH3��H2O�ĵ���ƽ�ⳣ��K=1.8��10-5��H2SO3�ĵ���ƽ�ⳣ��K1=1.2��10-2��K2=1.3��10-8����ͨ�����Ĺ����У���ǡ���γ�����ʱ����Һ������Ũ�ȵĴ�С��ϵΪ____��
����Ŀ����һ���������İ�������粒������ں��ݵ��ܱ���������У��ں㶨�¶���ʹ��ﵽ�ֽ�ƽ�⣺NH2COONH4��s�� ![]() 2NH3��g����CO2��g����ʵ���ò�ͬ�¶��µ�ƽ�����������±���
2NH3��g����CO2��g����ʵ���ò�ͬ�¶��µ�ƽ�����������±���
�¶�/�� | 15 | 20 | 25 | 30 | 35 |
ƽ����ѹǿ/kPa | 5.7 | 8.3 | 12.0 | 17.1 | 24.0 |
ƽ��������Ũ��/ 10-3mol��L��1 | 2.4 | 3.4 | 4.8 | 6.8 | 9.4 |
����˵����ȷ����
A. �÷�Ӧ�ڵ����¿����Է�����
B. ����ϵ�������ƽ����Է�����������ʱ��˵���÷�Ӧ�ﵽ��ƽ��״̬
C. ���������£����������ٳ���2mol NH3��1molCO2��ƽ�������ƶ���ƽ���NH3��Ũ�ȼ�С
D. 15��ʱ���÷�Ӧ��ƽ�ⳣ��ԼΪ2.05��10-9
����Ŀ�����ǻ�ѧʵ���Ҽ����������е���Ҫ���ʣ�Ӧ�ù㷺��
��1����֪25��ʱ��N2(g)��O2(g)![]() 2NO(g) ��H=+183 kJ/mol
2NO(g) ��H=+183 kJ/mol
2H2(g)��O2(g)��2H2O(l) ��H=��571.6 kJ/mol
4NH3(g)��5O2(g)��4NO(g)��6H2O(l) ��H=��1164.4 kJ/mol
��N2(g)��3H2(g)![]() 2NH3(g) ��H=______kJ/mol
2NH3(g) ��H=______kJ/mol
��2���ں��º����ܱ������н��кϳɰ���Ӧ����ʼͶ��ʱ������Ũ�����±���
N2 | H2 | NH3 | |
Ͷ�Ϣ� | 1.0 mol/L | 3.0 mol /L | 0 |
Ͷ�Ϣ� | 0.5 mol/L | 1.5 mol/L | 1.0 mol/L |
�ٰ�Ͷ�Ϣ���з�Ӧ����ôﵽ��ѧƽ��״̬ʱH2��ת����Ϊ40%������¶��ºϳɰ���Ӧ��ƽ�ⳣ������ʽΪ_______��
�ڰ�Ͷ�Ϣ���з�Ӧ����ʼʱ��Ӧ���еķ���Ϊ________�������������
���������¶ȣ���ϳɰ���Ӧ�Ļ�ѧƽ�ⳣ��________����������С�����䡱����
��L��L1��L2����X�ɷֱ����ѹǿ���¶ȡ���ͼ��ʾLһ��ʱ���ϳɰ���Ӧ��H2(g)��ƽ��ת������X�ı仯��ϵ��
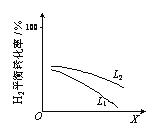
�� X��������������______��
�� �ж�L1��L2�Ĵ�С��ϵ������������______��
��3���绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ��ʾ��ͼ���£�
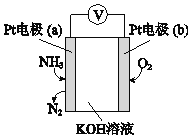
�ٵ缫b�Ϸ�������______��Ӧ�����������ԭ������
��д���缫a�ĵ缫��Ӧʽ_________��