��Ŀ����
����Ŀ����NAΪ����٤��������ֵ������˵����ȷ����
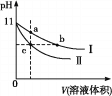
A. 2L 0.5mol/L��������Һ�к��е�H+������Ϊ2NA
B. �����£�1L pH=13��NaOH��Һ�У���ˮ�����OH-������ĿΪ0.1NA
C. ��⾫��ͭ�Ĺ����У�ÿת��NA������ʱ�������ܽ�ͭ������Ϊ32g
D. ij�ܱ�������ʢ��0.1mol N2��0.3mol H2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿС��0.6NA
���𰸡�D
��������
A��������Ϊ���ᣬˮ��Һ�в��ֵ��룻
B������������Һ�У�����������������ˮ�ĵ��룻
C����⾫��ͭʱ�������ϳ���ͭ�ŵ磬���б�ͭ���õĽ����ŵ磻
D���ϳ��ȵķ�ӦΪ���淴Ӧ��
A��2L0.5mol��L��1��������Һ�к��е�H��������С��2NA���ʲ��������⣻
B������������Һ�У�����������������ˮ�ĵ��룬��Һ�е���������ˮ����ģ���ˮ��������������Ũ��=������Ũ��![]() mol��L��1���ʲ��������⣻
mol��L��1���ʲ��������⣻
C����⾫��ͭʱ�������ϳ���ͭ�ŵ磬���б�ͭ���õĽ����ŵ磬�ʵ�ת��N4������ʱ����������������С��32g���ʲ��������⣻
D���ϳɵ��ķ�ӦΪ���淴Ӧ�����ܽ��г��ף���ת�Ƶĵ�����С��0.6N4�����ʷ������⣻��ѡ��D��

����Ŀ����ͼ��ʵ�����Ʊ���������֤�������ʵ�װ��(���мг�װ����ʡ��)��
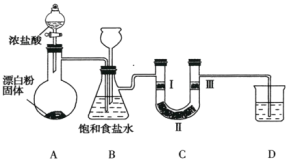
��֪��װ��A�������ķ���װ�ã���Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
�ݴ˻ش��������⣺
(1)װ��B�б���ʳ��ˮ��������_________��
(2)װ��BҲ�ǰ�ȫƿ��Ŀ���Ǽ��ʵ�����ʱװ��C���Ƿ�����������д��װ��C�з�������ʱװ��B�е�ʵ������__________________��
(3)װ��C����������֤�����Ƿ����Ư���ԣ���װ��C��I��������Ӧ�����������___________(����ĸ)��
��� | I | �� | �� |
a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
b | �������ɫ���� | ��ˮ����ͭ | ʪ�����ɫ���� |
c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
(4)װ��D��������_____________________________��
(5)�����20mL��10mol��L-1��Ũ����������������Ƴ�ַ�Ӧ��ʵ�����ռ����������ڱ�״���µ������__________��
A.��2.24 L B.��2.24 L C.��2.24 L D.��2.24 L