��Ŀ����
����Ŀ��t��ʱ,�����Ϊ2L���ܱ�������(�ݻ�����)ͨ��3mo1��1molB,������Ӧ3A(g)+B(g)xC(g);����2min��ʱ��÷�Ӧ�ﵽƽ��״̬(�����¶Ȳ���),ʣ���B�����ʵ���Ϊ0.8mol�����C��Ũ��Ϊ0.4 molL-1
��ش���������:
��1��������Ӧ������,B��ƽ����Ӧ����Ϊ______________
��2��x=_______,ƽ�ⳣ��K=_______.
��3��������ƽ����ϵ��ͨ����������������Ӧ��ϵ��ѹǿ,��ô��ѧƽ����ƶ�������____________.
��4�����¶Ȳ���,����ԭƽ������������г���amolC��t��ʱ�ﵽ�µ�ƽ��,��ʱB�����ʵ���Ϊnmol,��C�����ʵ���Ϊ_____(�ú�n�ı���ʽ��ʾ),ƽ�ⳣ��K______(��������������С������)
���𰸡� 0.05molL-1min-1 4 ![]() (
(![]() 0.037) ���ƶ� n ����
0.037) ���ƶ� n ����
������������3A(g)+B(g)xC(g);B��ƽ����Ӧ���ʿ�ֱ��������B�����ʵ���Ũ�ȵı仯ֵ����ʱ������⼴![]() ��= 0.05molL-1min-1
��= 0.05molL-1min-1
(2)C�����ʵ���n(C)��2 L��0.4 mol��L��1��0.8 mol����B�����ʵ����仯Ϊ0.2 mol������x��4���������A��B��C��Ũ�ȷֱ�Ϊc(A)��1.2 mol��L��1��c(B)��0.4 mol��L��1��c(C)��0.4 mol��L��1�����Դ���ƽ�ⳣ��ʽ�ɵ�ƽ�ⳣ��k=![]() ��
��
(3)���뺤�������ܷ�Ӧ��ϵ��ѹǿ���ӣ��������ʲ�����ϵ�е��������ʷ�Ӧ�����ᵼ����������Ũ�ȵı仯������ƽ�ⲻ���ƶ���
(4)�ﵽ�µ�ƽ��ʱA��B��C��ƽ��Ũ��֮�Ȳ��䣬��Ϊ1.2��0.4��0.4��3��1��1����n(C)��nmol��ƽ�ⳣ��K���䡣

 �Ķ��쳵ϵ�д�
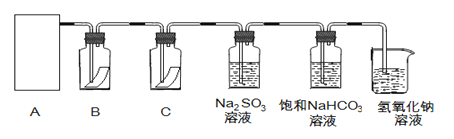
�Ķ��쳵ϵ�д�����Ŀ������ͼװ�ÿ������һϵ��ʵ�飨ͼ�мг�װ�ü�����װ������ȥ���������Ǣ٢ڢ������ʼ���Ӱ�졣��ش��������⣺
��1����װ��Aѡ��Ũ����Ͷ������̻����ȡCl2���壬װ��B�е������������������´���������д���пհף�
B������� | �� | �� | �� |
��պ�Լ� | ʯ����Һ | ����KI��Һ | Ũ��ˮ |
���� | ________ | ________ | ________ |
�漰�Ļ�ѧ����ʽ | ________ | ________ | 3Cl2+8NH3=6NH4Cl+N2 |
��2����װ��Aѡ��Ũ������������ƹ�����ȡSO2���壬װ��B�е������������������´���������д���пհף�
B������� | �� | �� | �� |
��պ�Լ� | H2S��Һ | ����KMnO4��Һ | Ʒ����Һ |
���� | ________ | ________ | ��ɫ |
����SO2������ | ________ | ________ | ________ |