��Ŀ����
����Ŀ������ͼʾ���Ӧ�������������

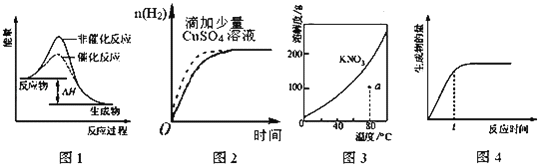
A. ͼ�ױ�ʾij���ȷ�Ӧ������(a)���д���(b)ʱ��Ӧ�������仯
B. ͼ�ұ�ʾ�����£�0.1mol/LNaOH��Һ�ζ�20.00mL0.1mol/L������Һ�ĵζ�����
C. ͼ����ʾij���淴Ӧ�ķ�Ӧ������ʱ��ı仯��ϵ��t0ʱ�̸ı��������ʹ���˴���
D. ͼ����ʾһ�������������ˮϡ�����С���Һ���������仯���ߣ��Ҵ������̶ȣ�a<b<c
���𰸡�D
��������
A��ͼ���з�Ӧ�����������������������������Ƿ��ȷ�Ӧ��ѡ��A����B���ζ��յ�ʱ���������Ƶ����Ϊ20.00mLʱ��Ϊ��������Һ����Һ�ʼ���pH>7��ѡ��B����C����Ӧǰ������������ȵķ�Ӧ���磺3A ��g��2C��g��+B��g��������ѹǿ���Сѹǿ��ƽ�ⲻ�ƶ�������ͼʾ����Ϊ����ѹǿ�������ܸı䷴Ӧ���ʣ�Ҳ����Ϊʹ���˴�����ѡ��C����D��������������ʣ����Դ�����Һ�д��ڵ���ƽ�⣬��ˮϡ�ʹٽ�������룬������Һ���Խ����ĵ���̶�Խ�����Դ������̶ȴ�С˳����c��b��a��ѡ��D��ȷ����ѡD��

����Ŀ�������ģ����������о���Ա������ú��������Ȼ�ѧѭ��ʵ��̫���ܵ�ת����洢���������£�

��1����Ӧ��2H2SO4(l)![]() 2SO2(g)+2H2O(g)+O2(g) ��H1=+551 kJ��mol��1
2SO2(g)+2H2O(g)+O2(g) ��H1=+551 kJ��mol��1
��Ӧ��S(s)+O2(g)![]() SO2(g) ��H3=��297 kJ��mol��1
SO2(g) ��H3=��297 kJ��mol��1
��Ӧ����Ȼ�ѧ����ʽ��________________��
��2���Է�Ӧ����ijһͶ�ϱ�ʱ������ѹǿ�£�H2SO4��ƽ����ϵ�����ʵ����������¶ȵı仯��ϵ��ͼ��ʾ��

p2_______p 1����������������ó��ý��۵�������________________��
��3��I��������Ϊˮ��Һ��SO2�绯��Ӧ�Ĵ��������ܵĴ��������¡���ii����������
i��SO2+4I��+4H+![]() S��+2I2+2H2O
S��+2I2+2H2O
ii��I2+2H2O+_________![]() _________+_______+2 I��
_________+_______+2 I��
��4��̽��i��ii��Ӧ������SO2�绯��Ӧ���ʵĹ�ϵ��ʵ�����£��ֱ�18 mL SO2������Һ���뵽2 mL�����Լ��У��ܱշ��ù۲�������֪��I2���ܽ���KI��Һ�У�
��� | A | B | C | D |
�Լ���� | 0.4 mol��L��1 KI | a mol��L��1 KI 0.2 mol��L��1 H2SO4 | 0.2 mol��L��1 H2SO4 | 0.2 mol��L��1 KI 0.0002 mol I2 |
ʵ������ | ��Һ��ƣ�һ��ʱ�����ֻ��� | ��Һ��ƣ����ֻ��ǽ�A�� | ���������� | ��Һ���غ�ɫ�ܿ���ɫ����ɻ�ɫ�����ֻ��ǽ�A�� |
��B��A�ĶԱ�ʵ�飬��a=__________��
�ڱȽ�A��B��C���ɵó��Ľ�����______________________��
��ʵ�������SO2���绯��Ӧ����D��A�����i��ii��Ӧ���ʽ���ԭ��________________��