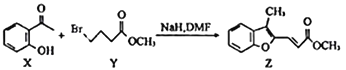��Ŀ����
����Ŀ��[��ѧ����ѡ��5���л���ѧ����
�л����㷺Ӧ���ں��ա��������ǣ����һ�ֺϳ�·�����£�
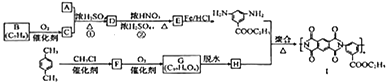
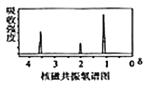
��֪�����л���A������ͼ��˴Ź�������ͼ���£�
��2RCOOH![]()
![]() +H2O
+H2O
��
�ش��������⣺
(1)A�Ļ�ѧ������________��C�к��������ŵ�������________��
(2)�ڵķ�Ӧ����Ϊ_________����ķ���ʽΪ_________��
(3)�ٵķ�Ӧ����ʽΪ_______________________________________��
(4)F�Ľṹ��ʽ��______________��
(5)ͬʱ��������������G��ͬ���칹�干��________��(��������ṹ)��д�����е�һ�ֽṹʽ��__________��
���ܷ���������Ӧ
���ܷ���ˮ�ⷴӦ����ˮ�����֮һ����FeCl3��Һ������ɫ��Ӧ
��1mol�������������8molNaOH��Ӧ
(6)д���ü���ױ��ͼ״�Ϊԭ���Ʊ�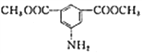 �ĺϳ�·�ߣ�________________(�����Լ���ѡ)��
�ĺϳ�·�ߣ�________________(�����Լ���ѡ)��
���𰸡� �� �Ȼ� ȡ����Ӧ (C19H10O6N2)n ![]() +C2H5OH
+C2H5OH![]()
![]() +H2O
+H2O ![]() 3
3 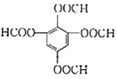
![]()
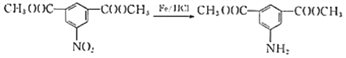
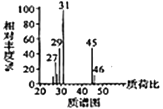
�������������������A������ͼ��֪����Է�������Ϊ46������˴Ź�������֪���������3��Hԭ�ӣ����Խ��I�Ľṹ��֪��AΪ�Ҵ����ɷ���ʽ�������е�ת����֪��BΪ![]() ����CΪ
����CΪ![]() ����A��C�ķ�Ӧ������֪DΪ
����A��C�ķ�Ӧ������֪DΪ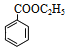 ���������Ӧ������E�ķ�Ӧ�����֪EΪ
���������Ӧ������E�ķ�Ӧ�����֪EΪ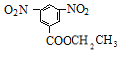 ������Ϣ�ۿ�֪��FΪ
������Ϣ�ۿ�֪��FΪ![]() ����F��G�ķ�Ӧ������G�ķ���ʽ��֪��GΪ
����F��G�ķ�Ӧ������G�ķ���ʽ��֪��GΪ![]() ��������Ϣ�ڿ�֪��HΪ
��������Ϣ�ڿ�֪��HΪ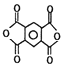 ��
��
��1��A�Ļ�ѧ�������Ҵ���CΪ�����ᣬ���еĺ������������Ȼ�.
��2���ڵķ�Ӧ����Ϊȡ����Ӧ����������Ӧ����I�ķ���ʽΪ(C19H10O6N2)n
��3���ٵķ�Ӧ����ʽΪ![]() ��
��
��4��F�Ľṹ��ʽ��![]() ��
��
��5�����ܷ���������Ӧ��˵����������ȩ�������ܷ���ˮ�ⷴӦ����ˮ�����֮һ����FeCl3��Һ������ɫ��Ӧ��˵����ˮ�����������з��ǻ�����1mol�������������8 mol NaOH��Ӧ��ͬʱ������Щ����G��ͬ���칹����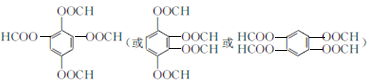 ������3����
������3����
��6���ü���ױ��ͼ״�Ϊԭ���Ʊ�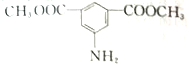 �������ȰѼ���ױ�����Ϊ��������ᣬȻ������������״���Ӧ�����ɼ����������������Ÿ��������е���Ϣ������������������������Ӧ������������ԭΪ�����õ���Ʒ������ϳ�·��������
�������ȰѼ���ױ�����Ϊ��������ᣬȻ������������״���Ӧ�����ɼ����������������Ÿ��������е���Ϣ������������������������Ӧ������������ԭΪ�����õ���Ʒ������ϳ�·��������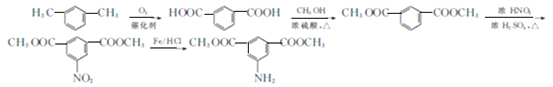 ��
��

����Ŀ������±��ش�������������Ϊ�����µ���������
�� | ���볣��(Ka) | �� | ���볣��(Ka) | �� | ���볣��(Ka) | �� | ���볣��(Ka) |
CH3COOH | 1.8��10-5 | H2CO3 | K1=4.4��10-7 K2=4.7��10-11 | H2C2O 4 | K1=5.4��10-2 K2=5.4��10-5 | H2S | K1=1.3��10-7 K2=7.1��10-15 |
HClO | 3��10-8 |
��ش��������⣺
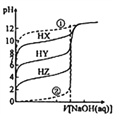
��1��ͬŨ�ȵ�CH3COO-��HCO3-��CO32-��HC2O4-��ClO-��S2-�н��H+��������������__________��
��2��������0.1 molL-1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����_______�����������
A.c(H+) B. c(H+)/c(CH3COOH) C. c(H+)/c(OH-) D. c(OH-)
��3��0.1 molL-1��H2C2O4��Һ��0.1 molL-1��KOH����Һ�������Ϻ�������Һ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ______________��
��4��pH��ͬ��NaClO��CH3COOK��Һ������Һ�����ʵ���Ũ�ȵĴ�С��ϵ�ǣ�CH3COONa______NaClO������Һ�У�[c(Na+)-c(ClO-)]______[c(K+)-c(CH3COO-)]���������������=������
��5����0.1 molL-1CH3COOH ��Һ�еμ� NaOH ��Һ�� c(CH3COOH): c(CH3COO-)=5 : 9����ʱ��ҺpH=_______________��