ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ≥ΘΈ¬ ±Θ§œύΆ§≈®Ε»ΒΡ»ΐ÷÷“Μ‘Σ»θΥα(HXΓΔHYΓΔHZ)ΓΔ«ΩΥαΓΔ¥ΩΥ°Ζ÷±π”ΟœύΆ§≈®Ε»ΒΡNaOH »ή“ΚΒΈΕ®,ΥυΒΟ»ή“ΚΒΡpH ”κΒΈ»κNaOH »ή“ΚΒΡΧεΜΐΙΊœΒ»γΆΦΥυ ΨΓΘ“―÷ΣΒΈΕ®Ιΐ≥Χ÷–¥φ‘ΎΘΚΒ±c(HX)=c(X-) ±,pH=10ΘΜc(HY)=c(Y- ) ±,pH=7;c(HZ)=c(Z-) ±,pH=4ΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
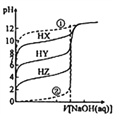
A. ΔΌΈΣ«ΩΥαΒΡΒΈΕ®«ζœΏ B. Υα–‘«Ω»θΘΚHX>HY>HZ
C. ΒΈΕ®HX»ή“ΚΩ…”ΟΦΉΜυ≥»Ής÷Η ΨΦΝ D. HZ+Y-![]() HY+Z-ΒΡΤΫΚβ≥Θ ΐΈΣK=1.0ΓΝ103
HY+Z-ΒΡΤΫΚβ≥Θ ΐΈΣK=1.0ΓΝ103
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩA. ΔΌΤπ ΦpH=7Θ§ΈΣ¥ΩΥ°Θ§Ι A¥μΈσΘΜ B. ΗυΨίc(HX)=c(X-) ±Θ§pH=10Θ§Φ¥Ka(HX)= 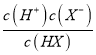 = c(H+)=10-10ΘΜΆ§άμKa(HY)=10-7ΘΜKa(HZ) =10-4Θ§K‘Ϋ¥σΘ§Υα–‘‘Ϋ«ΩΘ§“ρ¥ΥΥα–‘ΘΚHZ>HY>HXΘ§Ι B¥μΈσΘΜC. ΒΈΕ®HX»ή“Κ ±÷’Βψ»ή“Κ≥ Φν–‘Θ§≤ΜΡή”ΟΦΉΜυ≥»Ής÷Η ΨΦΝΘ§Ι C¥μΈσΘΜD. HZ+Y-
= c(H+)=10-10ΘΜΆ§άμKa(HY)=10-7ΘΜKa(HZ) =10-4Θ§K‘Ϋ¥σΘ§Υα–‘‘Ϋ«ΩΘ§“ρ¥ΥΥα–‘ΘΚHZ>HY>HXΘ§Ι B¥μΈσΘΜC. ΒΈΕ®HX»ή“Κ ±÷’Βψ»ή“Κ≥ Φν–‘Θ§≤ΜΡή”ΟΦΉΜυ≥»Ής÷Η ΨΦΝΘ§Ι C¥μΈσΘΜD. HZ+Y-![]() HY+Z-ΒΡΤΫΚβ≥Θ ΐΈΣK=
HY+Z-ΒΡΤΫΚβ≥Θ ΐΈΣK=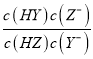 =
=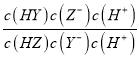 =
=![]() =1.0ΓΝ103Θ§Ι D’ΐ»ΖΘΜΙ ―ΓDΓΘ
=1.0ΓΝ103Θ§Ι D’ΐ»ΖΘΜΙ ―ΓDΓΘ

ΓΨΧβΡΩΓΩΦν Ϋ¬»Μ·ΟΨ(MgOHCl)≥Θ”ΟΉςΥήΝœΧμΦ”ΦΝΘ§Έ“Ιζ Ή¥¥άϊ”Ο«β―θΜ·Οؔꬻ̷οß»»Ζ÷Ϋβ÷ΤΑ±Τχ≤ΔΒΟΒΫΦν Ϋ¬»Μ·ΟΨΒΡΙΛ“’ΓΘΡ≥ΩΤ―––ΓΉιΗυΨίΗΟ‘≠άμ…ηΦΤΝΥ»γΆΦ Β―ιΉΑ÷Ο÷Τ±ΗΦν Ϋ―θΜ·ΟΨ≤ΔΧΫΨΩΑ±ΒΡΜΙ‘≠–‘Θ§Ζ¥”Π«ΑΘ§ΉΑ÷ΟC÷–CuOΒΡ÷ ΝΩΈΣ14.40gΓΘ
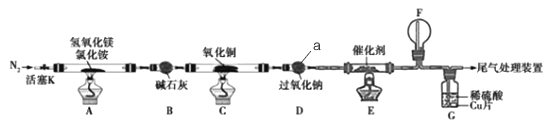
«κΜΊ¥πœ¬Ν–Έ Χβ:
(1)“«ΤςaΒΡΟϊ≥Τ «_______.
(2)ΉΑ÷ΟA÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________,ΉΑ÷ΟBΒΡΉς”Ο «_________,ΉΑ÷ΟDΒΡΉς”Ο «________
(3)ΉΑ÷ΟG÷–ΒΡœ÷œσΈΣ____________
(4)Ζ¥”ΠΫα χΚσΉΑ÷ΟC÷–ΒΡ―θΜ·Ά≠Άξ»ΪΖ¥”Π…ζ≥…Κλ…ΪΙΧΧεΘ§≤Δ≤βΒΟΤδ÷ ΝΩΈΣ12.24gΓΘ‘ρΚλ…ΪΙΧΧε «______(ΧνΜ·―ß Ϋ)Θ§ΗΟΖ¥”Π÷–ΉΣ“ΤΒγΉ”ΒΡΈο÷ ΒΡΝΩΈΣ_______mol.
(5)Άξ≥…œ¬Ν– Β―ιΖΫΑΗΘ§÷ΛΟςΉΑ÷ΟC÷–Άξ»ΪΖ¥”ΠΚσΒΟΒΫΒΡΚλ…ΪΙΧΧε÷–Κ§”–―θΜ·―«Ά≠(Ι©―Γ‘ώΒΡ ‘ΦΝ:2mol/LΒΡHNO3»ή“ΚΓΔ2mol/LΒΡH2SO4»ή“ΚΓΔ2mol/LΒΡNaOH»ή“Κ)ΓΘ
“―÷ΣΘΚCu2O+2H+=Cu2++Cu+H2O.
Β―ι≤Ϋ÷η | ‘ΛΤΎœ÷œσΚΆΫα¬έ |
≤Ϋ÷η1»ΓΉΑ÷ΟC÷–ΥυΒΟΚλ…ΪΙΧΧε”Ύ¥σ ‘Ιή÷– | ----- |
≤Ϋ÷η2:_____________________________ | _______________ |