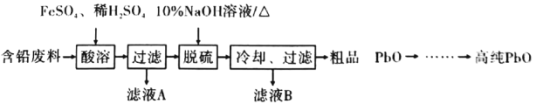
题目内容
【题目】化学是一把双刃剑,利用得好会给人类带来福祉,利用不好会给人类带来毁灭性的灾难。
(1)二战期间日本在战场上大量使用毒气弹里的毒气是芥子气,芥子气的结构简式为: ClCH2CH2-S-CH2CH2Cl。
①芥子气中四个碳原子是否在一条直线上________(填“是” 或“否”)。
②芥子气的核磁共振氢谱图上有________个吸收峰,其峰面积之比为________。
(2)苏合香醇可以用作食用香精,其结构简式如图所示:
![]()
①苏合香醇的分子式为________。
②下列物质不能与苏合香醇发生反应的是________ (选填序号)。
a.金属钠 b.HBr c.Na2CO3溶液 d.乙酸
③苏合香醇在铜作催化剂时发生氧化反应的化学方程式为________。
④苏合香醇发生消去反应的化学方程式为____________。
【答案】 否 2 1∶1 C8H10O c 2![]() +O2
+O2![]() 2
2![]() +2H2O
+2H2O ![]()
![]()
![]() +H2O
+H2O
【解析】(1)①ClCH2CH2-S-CH2CH2Cl中S和C均为sp3杂化,呈锯齿形;②芥子气有2种不同环境的氢,核磁共振氢谱图上有2个吸收峰;(2)苏合香醇的分子式为C8H10O,与-OH相连的碳上有氢,邻碳上也有氢,由-OH和苯环可知,能发生取代、加成、氧化、消去反应,而不能发生水解、加聚反应。
(1)①ClCH2CH2-S-CH2CH2Cl中S和C均为sp3杂化,呈锯齿形,芥子气中四个碳原子不在一条直线上;②分子中含有-S-,与硫原子相连的两个原子团完全相同,芥子气的核磁共振氢谱图上有2个吸收峰,其峰面积之比为4:4=1:1。(2)①苏合香醇的分子式为C8H10O;②由-OH和苯环的性质可知,能发生取代、加成、氧化、消去反应,而不能发生水解、加聚反应,a.-OH与金属钠发生取代反应,故正确;b.-OH与HBr发生取代反应,故正确;c.与Na2CO3溶液不反应;d.与乙酸发生酯化反应,故正确;故选c;③苏合香醇在铜作催化剂时发生氧化反应的化学方程式为 2![]() +O2
+O2![]() 2
2![]() +2H2O;④苏合香醇发生消去反应的化学方程式为
+2H2O;④苏合香醇发生消去反应的化学方程式为 ![]()
![]()
![]() +H2O。
+H2O。

【题目】如下表是元素周期表的短周期,请根据表中的位置,按要求回答下列问题:
族 周期 | ⅠA | ⅡA | ⅢA | ⅣA | ⅤA | ⅥA | ⅦA | 0族 |
1 | ① | |||||||
2 | ② | ③ | ④ | |||||
3 | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | ⑩ |
(1)用元素符号填空:在这十种元素中,化学性质最稳定的是_____,通常情况下化合价只有负价而无正价的是______,原子半径最小的是_______,原子半径最大的主族元素是_________。
(2)用化学式填空:在这十种元素中,最高价氧化物的水化物碱性最强的是______,酸性最强是________,呈两性的是_________。
(3)①和②形成的最简单的化合物的结构式是_____________。
(4)元素③的气态氢化物的电子式是________________。
(5)③可以形成多种氧化物,其中一种是红棕色气体,试用化学方程式说明该气体不宜采用排水法收集的原因__________________________。