��Ŀ����
�±���Ԫ�����ڱ���һ���֣�����A��G�ֱ����һ��Ԫ�ء�

����ݱ�������Ԫ�أ��ش��������⣺
��1������Ԫ���е�һ��������С���� ����Ԫ�ط��ţ���DԪ��ԭ�Ӻ����� �ֲ�ͬ�˶�״̬�ĵ��ӣ���̬ԭ�ӵļ۵��Ӳ��У�δ�ɶԵ���������Ԫ���� ����Ԫ�ط��ţ���
��2��AC2���ӵĿռ乹���� ���÷�����Aԭ�ӵ��ӻ���ʽΪ ��
��3��B����̬�⻯����ˮ�е��ܽ��Զ����A��C����̬�⻯����ܽ�ȣ�ԭ���� ��
��4����̬G2+�ĺ�������Ų�ʽ�� ���Ҷ������ṹ��ʽΪH2N��CH2һCH2��NH2�������е�̼ԭ�ӵ��ӻ���ʽΪ ��G2+���Ҷ������γ������� ���������к��еĻ�ѧ�������� ������ĸ��ţ���
���������к��еĻ�ѧ�������� ������ĸ��ţ���
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
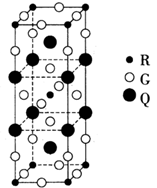
��5��������EF[F��AB��6]��һ�ֳ��������ʾ��壬���е�AB����B2Ϊ�ȵ����壬��AB���ĵ���ʽΪ ����ͼΪ����ɫ���徧���� ��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����
��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����


����ݱ�������Ԫ�أ��ش��������⣺
��1������Ԫ���е�һ��������С���� ����Ԫ�ط��ţ���DԪ��ԭ�Ӻ����� �ֲ�ͬ�˶�״̬�ĵ��ӣ���̬ԭ�ӵļ۵��Ӳ��У�δ�ɶԵ���������Ԫ���� ����Ԫ�ط��ţ���
��2��AC2���ӵĿռ乹���� ���÷�����Aԭ�ӵ��ӻ���ʽΪ ��
��3��B����̬�⻯����ˮ�е��ܽ��Զ����A��C����̬�⻯����ܽ�ȣ�ԭ���� ��
��4����̬G2+�ĺ�������Ų�ʽ�� ���Ҷ������ṹ��ʽΪH2N��CH2һCH2��NH2�������е�̼ԭ�ӵ��ӻ���ʽΪ ��G2+���Ҷ������γ�������
 ���������к��еĻ�ѧ�������� ������ĸ��ţ���
���������к��еĻ�ѧ�������� ������ĸ��ţ���a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
��5��������EF[F��AB��6]��һ�ֳ��������ʾ��壬���е�AB����B2Ϊ�ȵ����壬��AB���ĵ���ʽΪ ����ͼΪ����ɫ���徧����
 ��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����
��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����
��1��K 17 Fe
��2��ֱ���� SP
��3��NH3��H2O����֮����������CH4��H2S��H2O���Ӽ䲻�������
��4��1s22s22p63s23p63d9 SP3 abd
��5��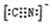 4
4
��2��ֱ���� SP
��3��NH3��H2O����֮����������CH4��H2S��H2O���Ӽ䲻�������
��4��1s22s22p63s23p63d9 SP3 abd
��5��
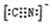 4
4 ���������
��1���������⣬A��C��B��N��C��S��D��Cl��E��K��F��Fe��G��Cu����һ������������K��D��17��Ԫ���ȣ���17�ֲ�ͬ�˶�״̬�ĵ��ӡ�Fe��δ�ɶԵ�����Ϊ4��
��2��CS2����CO2�Ľṹ��ͬ������Ҳ��ֱ���ͣ�SP�ӻ����͡�
��3��NH3��H2O����֮����������CH4��H2S��H2O���Ӽ䲻�������
��4��Cu2+�ĺ�������Ų�ʽ��1s22s22p63s23p63d9���Ҷ����е�̼ԭ���γɵ��ǵ������������ӻ���ʽΪ SP3���������еĻ�ѧ���зǼ��Լ������Լ���Cu2+��N֮������λ����
��5���ݡ�AB����B2Ϊ�ȵ����塱����CN-�ĵ�����ҲΪ14����ͼ֪��F2+Ϊ
 X4��F3+Ϊ
X4��F3+Ϊ X4��AB-Ϊ
X4��AB-Ϊ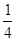 X12=3�����ݵ��������Ϊ0��ԭ����ɫ���徧����
X12=3�����ݵ��������Ϊ0��ԭ����ɫ���徧���� ��E+δ��������
��E+δ�������� ���е�E+Ϊ
���е�E+Ϊ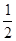 �������ɫ�����һ��������E+�ĸ���Ϊ8x1/2="4"
�������ɫ�����һ��������E+�ĸ���Ϊ8x1/2="4" 
��ϰ��ϵ�д�
�����Ŀ





 ��ÿ�������з�̯2����ԭ��
��ÿ�������з�̯2����ԭ�� Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��
Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��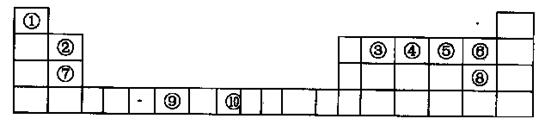
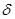 ����
����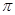 ���ĸ�����Ϊ ��
���ĸ�����Ϊ ��
