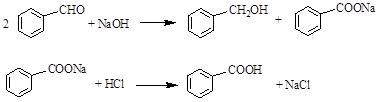
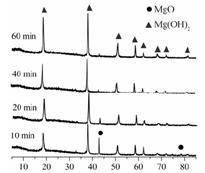
��Ŀ����
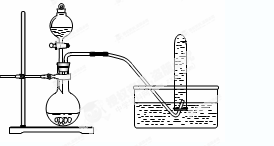
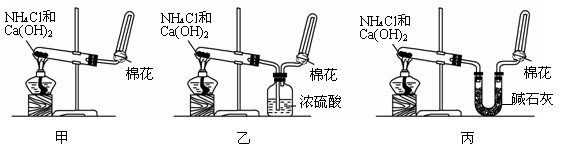
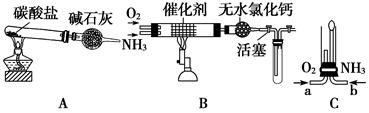
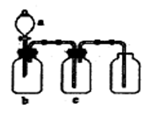
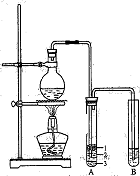
��ͼA��ʵ������ʯ��ʯ��ϡ������ȡCO2�ij���װ�á���ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ����ͼ�е�Dװ���ռ�һƿ���������CO2���塣
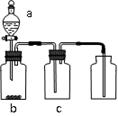
��1��a��b���������Ʒֱ��� �� ��
��2������D�������Ƿ��ռ����˵IJ����ǣ�_________________________________��
��3��B��װ��һ�����ı���NaHCO3Ŀ�ij�ȥA�ӷ���HCl��Ӧ�ķ���ʽ
��4��C��װ��������ȥˮ��������ѡ��������
aŨ���� b�ռ� c��ˮ�Ȼ��� d��ʯ��

��1��a��b���������Ʒֱ��� �� ��
��2������D�������Ƿ��ռ����˵IJ����ǣ�_________________________________��
��3��B��װ��һ�����ı���NaHCO3Ŀ�ij�ȥA�ӷ���HCl��Ӧ�ķ���ʽ
��4��C��װ��������ȥˮ��������ѡ��������
aŨ���� b�ռ� c��ˮ�Ȼ��� d��ʯ��
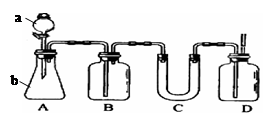
��1�� ��Һ©�� �� ��ƿ ��2��ȼ��ľ����������ƿ
��3�� NaHCO3+HCl=NaCl+H2O+CO2 ��4�� C
��3�� NaHCO3+HCl=NaCl+H2O+CO2 ��4�� C
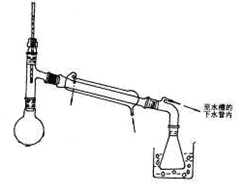
���������CO2���岻��ȼ�գ�����ȼ�ŵ�ľ����������ƿ����ľ��Ϩ��������������塣����NaHCO3��CO2����Ӧ���������ڳ��ӡ�Cװ��ΪU�ܣ�����װ����������CO2���������壬�ʲ������ռ�ͼ�ʯ�Ҹ��
�������Ի�ѧʵ��Ŀ���������ĸ߿��ص㣬�����ڱ�����Ӧע��Ի�ѧ������ʹ�á���ѧʵ������ʵ�鰲ȫ���������ʵ��Ʊ����ռ��ȵ����֪ʶ�Ļ��ۡ�֪ʶ��϶࣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
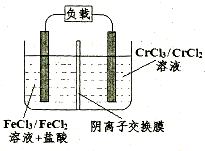
 ����������ҵ���Ʊ���ˮ��
����������ҵ���Ʊ���ˮ��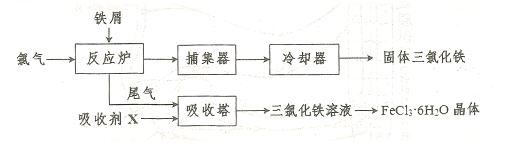
 Fe2++Cr3+�����طŵ�ʱ��Cl-������ ��������������������ʱ�������ĵ缫��ӦʽΪ ��
Fe2++Cr3+�����طŵ�ʱ��Cl-������ ��������������������ʱ�������ĵ缫��ӦʽΪ ��