��Ŀ����
I�ס��ҡ�����λͬѧ�ֱ�����������ʵ��װ�ü���ѧҩƷ�����м�ʯ��Ϊ�����������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش��������⣺
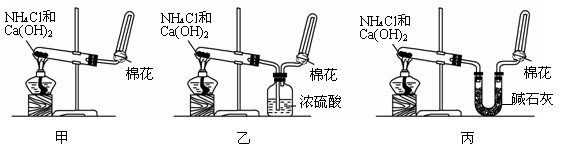
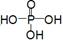
��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��_____________________________________________��
��2����λͬѧ������װ����ȡ����ʱ,������ͬѧû���ռ�������������ǵ�ʵ���������ȷ��������Ϊ�ռ�������������Ҫԭ����_____________________________________(�û�ѧ����ʽ��ʾ)��
��3�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ�_______________________
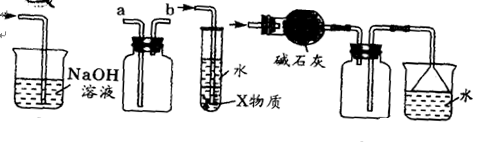
IIijУ��ѧС��ѧ�������ͼװ��(ͼ�����еȼг�װ������ȥ)���а�����������ʵ�顣
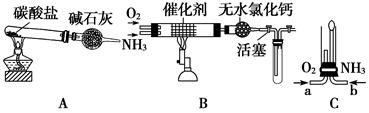
��4����װ��A��ȡ����������İ��������Թ�����̼���Σ���ʯ�ҵ�������__________________________________��
��5���������İ��������������ͨ��װ��B(����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ����������Ļ�ѧ����ʽ��_______________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��_________________��

��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��_____________________________________________��
��2����λͬѧ������װ����ȡ����ʱ,������ͬѧû���ռ�������������ǵ�ʵ���������ȷ��������Ϊ�ռ�������������Ҫԭ����_____________________________________(�û�ѧ����ʽ��ʾ)��
��3�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ�_______________________
IIijУ��ѧС��ѧ�������ͼװ��(ͼ�����еȼг�װ������ȥ)���а�����������ʵ�顣

��4����װ��A��ȡ����������İ��������Թ�����̼���Σ���ʯ�ҵ�������__________________________________��
��5���������İ��������������ͨ��װ��B(����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ����������Ļ�ѧ����ʽ��_______________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��_________________��
1��2NH4Cl + Ca(OH)2 ==CaCl2 + 2NH3��+ 2H2O
(2) 2NH3 + H2SO4 =(NH4)2SO4
��3����ʪ�ĺ�ɫʯ����ֽ���ڹܿڣ�����������
��4������CO2��ˮ����
��5��4NH3 + 5O2 ="==4NO" + 6H2O 2NO + O2 =2NO2
(2) 2NH3 + H2SO4 =(NH4)2SO4
��3����ʪ�ĺ�ɫʯ����ֽ���ڹܿڣ�����������
��4������CO2��ˮ����
��5��4NH3 + 5O2 ="==4NO" + 6H2O 2NO + O2 =2NO2
�����������1����λͬѧ���������Ȼ�����������Ʒ�Ӧ�Ʊ��������仯ѧ����ʽΪ
2NH4Cl + Ca(OH)2 ==CaCl2 + 2NH3��+ 2H2O����2�������ܹ���Ũ���ᷴӦ��������ͬѧ�ղ��������������������·�Ӧ��2NH3 + H2SO4 =(NH4)2SO4 ����3�����鰱���Ƿ��ռ����ķ����ǣ���ʪ�ĺ�ɫʯ����ֽ���ڹܿڣ���������������4����̼�������Ȼ�立�Ӧ�����˶�����̼��ˮ�����Լ�ʯ�ҵ�����������CO2��ˮ��������5�����������Ļ�ѧ����ʽ�ǣ�4NH3 + 5O2 ="==4NO" + 6H2O��NO���Ա���������Ϊ����ɫ�Ķ����������仯ѧ����ʽΪ2NO + O2 =2NO2��
���������⿼����ʵ�����Ʊ������Ļ���֪ʶ���Ǹ߿�������ص㣬���ⲻ�ѡ�

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ
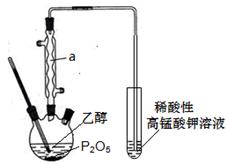

 ��__________________________________��
��__________________________________��

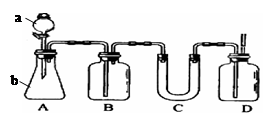
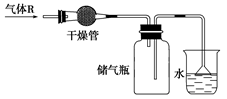
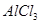 ������Һ�ô�����
������Һ�ô�����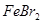 ��Һ�м����������ˮ������������
��Һ�м����������ˮ������������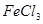 ����
����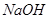 ��Һ���������ˡ�ϴ�ӳ������ٳ�����ճ�����
��Һ���������ˡ�ϴ�ӳ������ٳ�����ճ�����