��Ŀ����
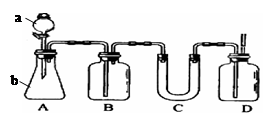
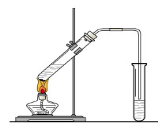
ʵ������ȡ�����������װ������ͼ��ʾ�������������������գ�
��Բ����ƿ�м���ķ�Ӧ�����廯�ơ� ��1:1�����ᡣ���������1��1���������õĶ�������Ϊ (ѡ���ţ���
a����ƽ b����Ͳ c������ƿ d���ζ���
b�����Թ�A�г��˲����ˮ֮�⣬�����ܴ��� �� (д����ѧʽ)��
�ǽ������ﵼ��ʢ�б�ˮ�������Թ�A�У���ˮ������������ ��
�Թ�A�е����ʷ�Ϊ���㣨��ͼ��ʾ���������ڵ� �㡣
��д������ʱ��ƿ�з�������Ҫ��Ӧ�Ļ�ѧ���� ʽ�� ��
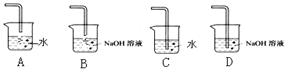
����Ũ���������ʵ�飬���Թ�A�л�õ��л�����ػ�ɫ����ȥ�������ʵ���ȷ�����ǣ�
(ѡ���ţ���
a������ b������������Һϴ�� c�������Ȼ�̼��ȡ d��������������Һϴ��
���Թ�B�е����Ը��������Һ��ɫ��ʹ֮��ɫ�����ʵ������� ��
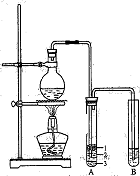
��ʵ��Ա��ʦ���������װ���е��������Ӳ��ֶ��ijɱ������ӿڣ���ԭ���ǣ� ��

��Բ����ƿ�м���ķ�Ӧ�����廯�ơ� ��1:1�����ᡣ���������1��1���������õĶ�������Ϊ (ѡ���ţ���
a����ƽ b����Ͳ c������ƿ d���ζ���
b�����Թ�A�г��˲����ˮ֮�⣬�����ܴ��� �� (д����ѧʽ)��
�ǽ������ﵼ��ʢ�б�ˮ�������Թ�A�У���ˮ������������ ��
�Թ�A�е����ʷ�Ϊ���㣨��ͼ��ʾ���������ڵ� �㡣
��д������ʱ��ƿ�з�������Ҫ��Ӧ�Ļ�ѧ���� ʽ�� ��
����Ũ���������ʵ�飬���Թ�A�л�õ��л�����ػ�ɫ����ȥ�������ʵ���ȷ�����ǣ�
(ѡ���ţ���
a������ b������������Һϴ�� c�������Ȼ�̼��ȡ d��������������Һϴ��
���Թ�B�е����Ը��������Һ��ɫ��ʹ֮��ɫ�����ʵ������� ��
��ʵ��Ա��ʦ���������װ���е��������Ӳ��ֶ��ijɱ������ӿڣ���ԭ���ǣ� ��
��12�֣�
�� �Ҵ� ��1�֣��� b ��1�֣�
�� HBr ��C2H5OH ��1��)
�� ��ȴ��Һ�������� ����1�֣�
3 ����1�֣�
��4�� NaBr+H2SO4�THBr+NaHSO4 ;��2�֣�
HBr+CH3CH2OH CH3CH2Br+H2O��2�֣�
CH3CH2Br+H2O��2�֣�
�� d ��1�֣�
��ϩ ��1�֣���
�� ��Ӧ���ܻ����Br2����ʴ��1�֣�
�� �Ҵ� ��1�֣��� b ��1�֣�
�� HBr ��C2H5OH ��1��)
�� ��ȴ��Һ�������� ����1�֣�
3 ����1�֣�
��4�� NaBr+H2SO4�THBr+NaHSO4 ;��2�֣�
HBr+CH3CH2OH
 CH3CH2Br+H2O��2�֣�
CH3CH2Br+H2O��2�֣��� d ��1�֣�
��ϩ ��1�֣���
�� ��Ӧ���ܻ����Br2����ʴ��1�֣�
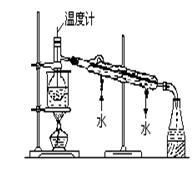
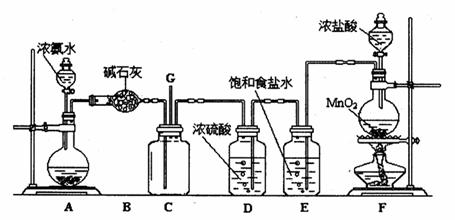
�����������1��ʵ������ȡ���������������Լ����Ҵ����廯�ⷴӦ�������ڼ����·���ȡ����Ӧ���������飻��ʵ����ȡ��Һ�����Ҫ��ȷ�Ȳ��Ǻܸߣ�������Ͳ��ȡ���ʴ�Ϊ���Ҵ��� b��
��2��ҩƷ��Ϻ��ڼ�������������HBr�����Ҵ�����ȡ����Ӧ���ʴ�Ϊ��NaBr+H2SO4�THBr+NaHSO4��HBr+CH3CH2OH
 CH3CH2Br+H2O��
CH3CH2Br+H2O����3���ռ�װ�����õ����ܽϳ�����������ȴ�����ã����ɵ��������ˮ�������ܣ�������������ܶȱ�ˮ���ڱ�ˮ�������²㣬�ʴ�Ϊ����ȴ��Һ�������飻 3��
��4���ڼ��������·�Ӧ��HBr��CH3CH2OH���ӷ��������������д���HBr��CH3CH2OH���ʴ�Ϊ��HBr��CH3CH2OH��
��5���Թ�A�л�õ��л�����ػ�ɫ��������Ũ�������ǿ�����ԣ���HBr������Br2������Ϊ�����飬���е����壬��������ȫ��ȥ���ʣ����Ҳ����鷳������������Һ��ʹ������ˮ�⣬���Ȼ�̼�������µ����ʣ����������ƺ��巢��������ԭ��Ӧ����HBr�������ƣ�������������룮���ڼ����¶Ƚϸ�ʱ���Ҵ�������ȥ��Ӧ������ϩ��
�ʴ�Ϊ��d�� ��ϩ��
��6��Ũ�������ǿ�����ԣ���HBr������Br2�����ɵ�Br2����ǿ�����ԣ��ḯʴ��Ӧ�ò������ܣ��ʴ�Ϊ����Ӧ�����Br2����ʴ��
������������Ҫ������������Ʊ����ᴿ���л���Ӧ�ȸ������Լ�ʵ�鰲ȫ�ȣ���1��2��3�ʣ�Դ�ڿα��жԸ���ʾʵ���ʵ�鱨�棬�ر��ǵ�6���С��������ӿڡ���һװ�ã����û�������о����̲ģ��Ͳ������Ϥ��װ�ã�����ͬѧ�Ǿ���ѡ��һЩȡ֮�ڽ̲ģ����ָ��ڽ̲ĵ�ϰ�⣮������Ĵ𰸾��ڽ̲�֮�У��������ӽ̲ľͺ���ȡ�úóɼ���

��ϰ��ϵ�д�
�����Ŀ