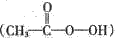��Ŀ����
Ϊ�����������ȱ�������ҹ������涨ʳ���α����Ǽӵ�ʳ�Σ���Ƶ��Ρ���ν���ξ�����ʳ���м���һ�����ĵ���أ�KIO3��������㣨��ȷ��0.1��:
(1)������е�Ԫ�ص�����������
(2)������ÿ��ʳ��7 g���Σ�������ȡ0.15 mg�⣬��ô1 kg�����к�����ٺ��ˣ�
(3)�ҹ��涨ÿǧ��ʳ���е���صĺ�����0.06��0.08 g��ij�о�С���ȡʳ����Ʒ100 g�����������⻯�ط�����Ӧ��KIO3+5KI+3H2SO4====3K2SO4+3I2+3H2O��������ɵⵥ��0.0254 g,�����1 000 g��Ʒ��KIO3�ĺ��������ж���Ʒ�Ƿ���Ϲ��ұ���
(1)������е�Ԫ�ص�����������
(2)������ÿ��ʳ��7 g���Σ�������ȡ0.15 mg�⣬��ô1 kg�����к�����ٺ��ˣ�
(3)�ҹ��涨ÿǧ��ʳ���е���صĺ�����0.06��0.08 g��ij�о�С���ȡʳ����Ʒ100 g�����������⻯�ط�����Ӧ��KIO3+5KI+3H2SO4====3K2SO4+3I2+3H2O��������ɵⵥ��0.0254 g,�����1 000 g��Ʒ��KIO3�ĺ��������ж���Ʒ�Ƿ���Ϲ��ұ���
��1��59.3% (2)21.4 mg (3)0.07g ����
��ʾ����1��w(I)=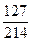 ��100%=59.3%
��100%=59.3%
(2)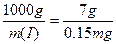 ����w(I)="21.4" mg
����w(I)="21.4" mg
(3)KIO3 �� 3I2
214 g 3��254 g
m(KIO3) 0.0254 g
��100 g������Ʒ��m(KIO3)="0.007" g
��1 000 g�����к�KIO3������Ϊ0.07 g,����0.06��0.08 g,�ʷ��Ϲ��ұ���
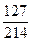 ��100%=59.3%
��100%=59.3%(2)
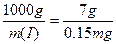 ����w(I)="21.4" mg
����w(I)="21.4" mg(3)KIO3 �� 3I2
214 g 3��254 g
m(KIO3) 0.0254 g
��100 g������Ʒ��m(KIO3)="0.007" g
��1 000 g�����к�KIO3������Ϊ0.07 g,����0.06��0.08 g,�ʷ��Ϲ��ұ���

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
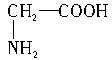 ��
��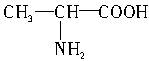
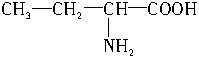 ����ͬϵ����̼Ԫ�ص��������������ֵ�ӽ��ڣ� ��
����ͬϵ����̼Ԫ�ص��������������ֵ�ӽ��ڣ� ��