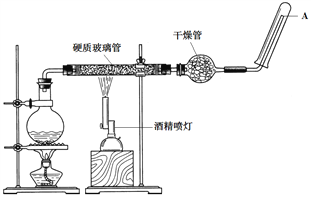
��Ŀ����
����Ŀ��ijFe 2(SO4) 3��Ʒ��������FeSO4���ʣ�Ϊ�˲ⶨ����Ʒ����Ԫ�صĺ������������ʵ�飺
��ȡ��Ʒm g������ϡH2SO4�����ˮ���Ƴ�250.00 mL��Һ��
��ȡ25.00 mL��Һ���ȼ���H2O2��Ȼ���ټӹ����İ�ˮ�����ˣ�
������������ˮϴ�����κ�ɣ�
�����������������ټ���Ϊֹ���õ�����ɫ���壬��ȴ�����������Ϊn g��
���������������̣��ش��������⣺
�ٲ�����г���ʹ�õ�������________________�������õ����������ձ�������������ͷ�ιܣ���Ҫ����IJ���������____________________��
�ڲ�����й����õ���������ֽ������̨����Ȧ���ձ�����Ҫ����IJ���������________������H2O2����ҪĿ����_______________________________��
����Ҫ������Һ�е�Fe3+����Ӧ�ü���________________�Լ���
�ܲ�����м���ó����Ѿ�ϴ�Ӹɾ��IJ�����________________��
�ݼ������Ʒ����Ԫ�ص����������ı���ʽ��________________��
���𰸡�������ƽ 250mL����ƿ©���Ͳ�������Fe2+����ΪFe3+KSCNȡ���һ��ϴ��Һ�������Թ��У��μ������ữ��BaCl2��Һ(��BaCl2��Һ)�����ް�ɫ����������֤����ϴ�Ӹɾ�7n/m��100%��700n/m%
��������
�ٲ�����г���Fe 2(SO4) 3��Ʒ������ʹ��������ƽ������250.00 mL��Һ���õ����������ձ�������������ͷ�ι���250mL����ƿ���ʴ�Ϊ��������ƽ��250mL����ƿ��
�ڹ����õ���������ֽ������̨����Ȧ���ձ�����Ҫ����IJ���������©���Ͳ�����������H2O2���Խ�Fe2+����ΪFe3+���ʴ�Ϊ��©���Ͳ���������Fe2+����ΪFe3+��
��Ҫ������Һ�е�Fe3+������ѡ��KSCN������KSCN����Һ��Ѫ��ɫ���ʴ�Ϊ��KSCN��
�ܲ�����еij���Ϊ������������Һ�е�����Ϊ(NH4)2SO4������ó����Ѿ�ϴ�Ӹɾ�����ͨ�������Ƿ����������������ɣ�����Ϊ��ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ��BaCl2��Һ(��BaCl2��Һ)�����ް�ɫ����������֤����ϴ�Ӹɾ����ʴ�Ϊ��ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ��BaCl2��Һ(��BaCl2��Һ)�����ް�ɫ����������֤����ϴ�Ӹɾ���
�����ĺ���ɫ����Ϊ������������Ϊng�����е���Ԫ�ص����ʵ���Ϊ![]() ��2=
��2=![]() mol������Ʒ����Ԫ�ص�����Ϊ
mol������Ʒ����Ԫ�ص�����Ϊ![]() mol��
mol��![]() ��56g/mol=7ng������Ʒ����Ԫ�ص���������=
��56g/mol=7ng������Ʒ����Ԫ�ص���������=![]() ��100%=
��100%=![]() ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ��![]() ��100%��
��100%��

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д�����Ŀ��������ͼ��ʾ��װ���У���ƿ�г�����������a�����ι��е�Һ��b������ƿ�ڣ���������ƿ��Ȼ����ɼ�f���ձ��е�Һ��b����Ȫ״��������ռ���������ƿ����a��b��������ĿҪ�����
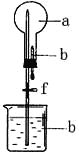
ѡ�� | a���������壩 | b��Һ�壩 |
A | Cl2 | ����NaOH��Һ |
B | SO2 | 4mol/LNaOH��Һ |
C | NO2 | ˮ |
D | NH3 | 1mol/L���� |
A. A B. B C. C D. D