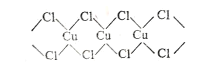
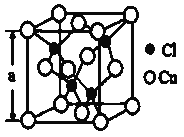
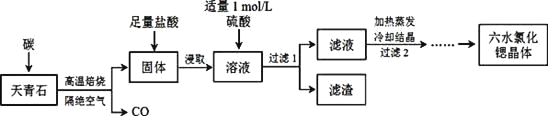
��Ŀ����
����Ŀ��һ�����缫���ˮ�����װ����ͼ�����缫Ϊ���缫a�����缫b��Ni(OH)2�缫��ͨ�����ƿ�������K1��K2���ɽ���õ�H2��O2������˵��������ǣ� ��
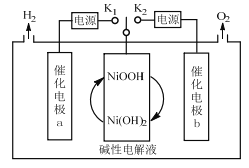
A.��O2ʱ��������Ni(OH)2�缫ͨ�����·������缫b
B.��H2ʱ�������ĵ缫��ӦʽΪNi(OH)2+OH--e-=NiOOH+H2O
C.���缫b�ϣ�OH-����������Ӧ����O2
D.��װ�ÿ�����Ĥ���������Ʊ��ߴ�����
���𰸡�A
��������
A�����缫b�У�ˮʧ��������O2���������������ɴ��缫bͨ�����·����Ni(OH)2�缫��A����
B����H2ʱ�����缫aΪ����������Ni(OH)2�ڼ�����Һ��ʧ��������NiOOH���缫��ӦʽΪNi(OH)2+OH--e-=NiOOH+H2O��B��ȷ��
C�����缫b�ϣ�ˮ���������OH-ʧ���ӣ�����������Ӧ����O2��C��ȷ��
D����װ���У������ֻ��ˮ�����Կ�����Ĥ���������Ʊ��ߴ�������D��ȷ��
��ѡA��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ