ЬтФПФкШн
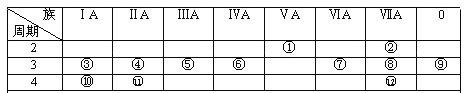
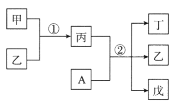
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЁЂEЮЊЖЬжмЦкдЊЫиЃЌЧвдзгађЪ§вРДЮдіДѓЃЌжЪзгЪ§жЎКЭЮЊ39ЃЌBЁЂCЭЌжмЦкЃЌAЁЂDЭЌжїзхЃЌAЁЂCФмаЮГЩСНжжвКЬЌЛЏКЯЮяA2CКЭA2C2ЃЌEдЊЫиЕФжмЦкађЪ§гыжїзхађЪ§ЯрЕШЃЌдЊЫиFгыCаЮГЩвЛжжОпгаДХадЕФЮяжЪGЁЃ
ЃЈ1ЃЉFдЊЫидкжмЦкБэжаЕФЮЛжУЮЊ___ЃЛBC2ЕФЕчзгЪНЮЊ___ЁЃ
ЃЈ2ЃЉгЩAЁЂBСНжждЊЫизщГЩЕФ18ЕчзгЮЂСЃЕФЗжзгЪНЮЊ___ЁЃ
ЃЈ3ЃЉЩЯЪідЊЫиЕФЦјЬЌЧтЛЏЮяжаЃЌЮШЖЈадзюЧПЕФЪЧ___ЃЈЬюЛЏбЇЪНЃЌЯТЭЌЃЉЃЛзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЪЧСНадЛЏКЯЮяЕФЪЧ___ЃЌЦфЪмШШЗжНтКѓЕФЙЬЬЌВњЮяПЩШмгкDЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяжаЃЌЦфЛЏбЇЗНГЬЪНЮЊ___ЁЃ
ЃЈ4ЃЉЗЯгЁЫЂЕчТЗАхЩЯКЌгаЭЃЌгУA2C2КЭЯЁСђЫсНўХнЗЯгЁЫЂЕчТЗАхПЩвдШмНтЭЃЌаДГіЗДгІЕФРызгЗНГЬЪН___ЃЛаДГіGгыЯЁЯѕЫсМгШШЗДгІЕФРызгЗНГЬЪН___ЁЃ
ЃЈ5ЃЉдЊЫиDЕФЕЅжЪдквЛЖЈЬѕМўЯТЃЌФмгыAЕЅжЪЛЏКЯЩњГЩвЛжжРызгЛЏКЯЮяDAЃЌШлЕуЮЊ800ЁцЃЌШєНЋ1molDAКЭ1molEЕЅжЪЛьКЯМгШызуСПЕФЫЎЃЌГфЗжЗДгІКѓЩњГЩЕФЦјЬхдкБъзМзДПіЯТЕФЬхЛ§ЪЧ___ЁЃ
ЁОД№АИЁПЕкЫФжмЦкЕкVIIIзх ![]() C2H6 H2O Al(OH)3 Al2O3+2NaOH=2NaAlO2+H2O H2O2+Cu+2H+=Cu2++2H2O 3Fe3O4+28H++NO3-
C2H6 H2O Al(OH)3 Al2O3+2NaOH=2NaAlO2+H2O H2O2+Cu+2H+=Cu2++2H2O 3Fe3O4+28H++NO3-![]() 9Fe3++NOЁќ+14H2O 56L
9Fe3++NOЁќ+14H2O 56L
ЁОНтЮіЁП
AЁЂBЁЂCЁЂDЁЂEЮЊЖЬжмЦкдЊЫиЃЌЧвдзгађЪ§вРДЮдіДѓЃЌAЁЂCФмаЮГЩСНжжвКЬЌЛЏКЯЮяA2CКЭA2C2ЃЌдђAЮЊHЁЂCЮЊOЃЌAЁЂDЭЌжїзхЃЌдђDЮЊNaЃЌEдЊЫиЕФжмЦкађЪ§гыжїзхађЪ§ЯрЕШЃЌдђEЮЊAlЃЌAЁЂBЁЂCЁЂDЁЂEжЪзгЪ§жЎКЭЮЊ39ЃЌBЁЂCЭЌжмЦкЃЌдђBжЪзгЪ§ЮЊ6ЃЌBЮЊCЃЌдЊЫиFгыCаЮГЩвЛжжОпгаДХадЕФЮяжЪGЃЌдђFЮЊFeЃЌGЮЊЫФбѕЛЏШ§ЬњЃЌОнДЫЛиД№ЃЛ
(1)FдЊЫиМДЬњдкжмЦкБэжаЕФЮЛжУЮЊЕкЫФжмЦкЕкVIIIзхЃЛBC2МДЖўбѕЛЏЬМЃЌЪєгкЙВМлЛЏКЯЮяЃЌЕчзгЪНЮЊ![]() ЃЛ
ЃЛ
Д№АИЮЊЃКЕкЫФжмЦкЕкVIIIзхЃЛ![]() ЃЛ
ЃЛ
(2)гЩAМДЬМЁЂBМДЧтСНжждЊЫизщГЩЕФ18ЕчзгЮЂСЃЕФЗжзгЪНЮЊC2H6ЃЛ
Д№АИЮЊЃКC2H6ЃЛ
(3ЃЉЗЧН№ЪєаддНЧПЦфЦјЬЌЧтЛЏЮядНЮШЖЈЃЌдђЩЯЪідЊЫиЕФЦјЬЌЧтЛЏЮяжаЃЌЮШЖЈадзюЧПЕФЪЧH2OЃЛзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЪЧСНадЛЏКЯЮяЕФЪЧЧтбѕЛЏТСЃЌDМДФЦЃЌЦфзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЮЊNaOHЃЌЧтбѕЛЏТСЪмШШЗжНтКѓЕФЙЬЬЌВњЮяЮЊбѕЛЏТСЃЌПЩШмгкNaOHШмвКЃЌЦфЛЏбЇЗНГЬЪНЮЊAl2O3+2NaOH=2NaAlO2+H2OЃЛ
Д№АИЮЊЃКH2OЃЛAl(OH)3ЃЛAl2O3+2NaOH=2NaAlO2+H2OЃЛ
(4) A2C2ЮЊH2O2ЃЌЫќКЭЯЁСђЫсНўХнЗЯгЁЫЂЕчТЗАхПЩвдШмНтЭЃЌЪЧвђЮЊЗЂЩњСЫбѕЛЏЛЙдЗДгІЃЌЭБЛбѕЛЏЃЌЫЋбѕЫЎБЛЛЙдЃЌЗДгІЕФРызгЗНГЬЪНЮЊH2O2+Cu+2H+=Cu2++2H2OЃЛGЮЊFe3O4ЃЌЯЁЯѕЫсОпЧПбѕЛЏадЃЌЭЈГЃЦфЛЙдВњЮяЮЊNOЃЌдђFe3O4гыЯЁЯѕЫсЗЂЩњбѕЛЏЛЙдЗДгІЃЌМгШШЗДгІЕФРызгЗНГЬЪНЮЊЃК3Fe3O4+28H++NO3-![]() 9Fe3++NOЁќ+14H2OЃЛ
9Fe3++NOЁќ+14H2OЃЛ
Д№АИЮЊЃКH2O2+Cu+2H+=Cu2++2H2OЃЛ3Fe3O4+28H++NO3-![]() 9Fe3++NOЁќ+14H2OЃЛ
9Fe3++NOЁќ+14H2OЃЛ
(5)дЊЫиDЕФЕЅжЪ(МДФЦ)дквЛЖЈЬѕМўЯТЃЌФмгыAЕЅжЪ(МДЧтЦј)ЛЏКЯЩњГЩЕФРызгЛЏКЯЮяЮЊNaHЃЌNaHКЭЫЎЗДгІЕФЗНГЬЪНЮЊNaH+H2O=NaOH+H2ЁќЃЌдђИУЗДгІжаЯћКФ1molNaHЩњГЩ1molNaOHКЭ1molH2ЃЌШєНЋ1molNaHКЭ1molAlЛьКЯМгШызуСПЕФЫЎЃЌСэЭтЛЙЗЂЩњЗДгІЕФЗНГЬЪНЮЊЃК2Al+2NaOH+2H2O=2NaAlO2+3H2ЁќЃЌдђгжПЩЕУ1.5mol H2ЃЌЙЪГфЗжЗДгІКѓЩњГЩ2.5mol H2ЃЌЙЪЦјЬхдкБъзМзДПіЯТЕФЬхЛ§ЪЧ56LЃЛ
Д№АИЮЊЃК56LЁЃ

 аЁЬьВХПЮЪБзївЕЯЕСаД№АИ
аЁЬьВХПЮЪБзївЕЯЕСаД№АИ вЛПЮЫФСЗЯЕСаД№АИ
вЛПЮЫФСЗЯЕСаД№АИ ЛЦИдаЁзДдЊТњЗжГхДЬЮЂВтбщЯЕСаД№АИ
ЛЦИдаЁзДдЊТњЗжГхДЬЮЂВтбщЯЕСаД№АИ аТИЈНЬЕМбЇЯЕСаД№АИ
аТИЈНЬЕМбЇЯЕСаД№АИ бєЙтЭЌбЇвЛЯпУћЪІШЋгХКУОэЯЕСаД№АИ
бєЙтЭЌбЇвЛЯпУћЪІШЋгХКУОэЯЕСаД№АИ