��Ŀ����
����Ŀ��ij��ɫ��Һ�к���K����Cl����OH����SO32-��SO42-��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
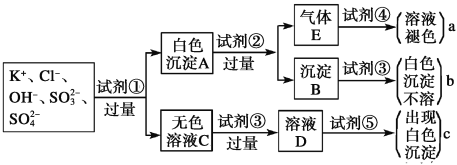
(1)ͼ���Լ��١������ʵĻ�ѧʽ�ֱ��ǣ�
��________����________����________����__________����__________��
(2)ͼ������c�����������������________________��
(3)��ɫ����A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����____________________��
(4)����Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��_________________________��
���𰸡�Ba(NO3)2 HCl HNO3 Br2 AgNO3 Cl�� ��ʹSO32����SO42���ļ���������ţ�����ȷ��SO42���Ƿ���� SO2��Br2��2H2O=4H����SO42����2Br��
��������
���ݿ�ͼ�������Ҫ��������ӿ�֪������Eֻ��Ϊ��������SO32-��SO42-�ܹ���Ba(NO3)2��Һ��Ӧ�ֱ����������ᱵ�����ᱵ��ɫ�����������ᱵ�����ᷴӦ�ܹ����ɶ����������壬������������E��ʹ��ˮ��ɫ�����ᱵ���ܽ������ᣬ���Լ���ΪBa(NO3)2��Һ���Լ���Ϊ���ᣬ�Լ���Ϊ��ˮ����ɫ��ҺC�к���Cl����OH������Һ�ʼ��ԣ���������Լ��ۺ��ټ����Լ������ɰ�ɫ������Ӧ���Ǽ��������ӣ�����Լ���Ϊ���ᣬ������Һ�����ԣ��Լ���Ϊ��������Һ����ɫ����Ϊ�Ȼ������ݴ˷������
(1)SO32-��SO42-�ܹ���Ba(NO3)2��Һ��Ӧ�ֱ����������ᱵ�����ᱵ��ɫ�����������ᱵ�����ᷴӦ�ܹ����ɶ����������壬������������E��ʹ��ˮ��ɫ�����ᱵ���ܽ������ᣬ���Լ���ΪBa(NO3)2��Һ���Լ���Ϊ���ᣬ�Լ���Ϊ��ˮ����ɫ��ҺC�к���Cl����OH������Һ�ʼ��ԣ���������Լ��ۺ��ټ����Լ������ɰ�ɫ������Ӧ���Ǽ��������ӣ�����Լ���Ϊ���ᣬ������Һ�����ԣ��Լ���Ϊ��������Һ����ɫ����Ϊ�Ȼ������ʴ�Ϊ��Ba(NO3)2��HCl��HNO3��Br2��AgNO3��
(2)������������������a�������������ΪSO32-������b�������������ΪSO42-������c�������������ΪCl-���ʴ�Ϊ��Cl-��
(3)�������ǿ�����ԣ��ܹ����������������������������ӣ���˰�ɫ����A�����Լ���ϡ����������Լ��������ʹSO32-��SO42-�ļ���������ţ�����ȷ��SO42-�Ƿ���ڣ��ʴ�Ϊ����ʹSO32-��SO42-�ļ���������ţ�����ȷ��SO42-�Ƿ���ڣ�
(4)�嵥�����������Ӧ��������������ᣬ��Ӧ�����ӷ���ʽ��Br2+SO2+2H2O=4H++SO42-+2Br-���ʴ�Ϊ��Br2+SO2+2H2O=4H++SO42-+2Br-��

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�����Ŀ�����ʵ�飬�������������������Ž��м��顣
(1)Ϊ��֤���������д�����ԭ�ӣ�ijͬѧ�������ʵ�飺
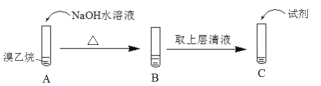
�� ���Թ�C�м�����Լ�����Ӧ��ʵ������������_______��
�� ��������NaOHˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��______��
(2)�û�ѧ��������![]() ���еĹ����š�
���еĹ����š�
�� ����±���
������ | �����Լ� | ʵ������ | ��ѧ����ʽ |
��OH | FeCl3��Һ | ��Һ����ɫ |
|
��COOH | _______ | ����ɫ���ݲ��� | _______ |
�� ��������ˮ�����Ƿ���̼̼˫�����������ɣ�_______��