��Ŀ����
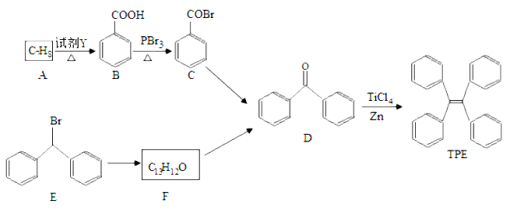
����Ŀ������ѧ��ѡ��5���л���ѧ�������˹��ϳ��л�������H��·�߿ɼ�ʾ���£�
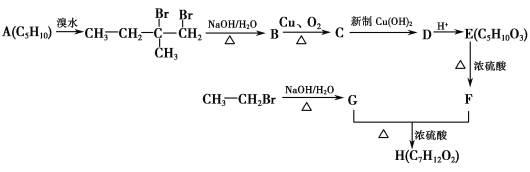
��֪��F�ĺ˴Ź����������ĸ��壬��������Ϊ1��1��3��3��
��ش��������⣺
��1��A������(ϵͳ����)Ϊ________��C�й����ŵĽṹ��ʽΪ_______________________��
��2��G��F�D��H�ķ�Ӧ����Ϊ________��H�Ľṹ��ʽΪ________��
��3��C�D��D�����ӷ���ʽΪ___________________________________________ ��
��4��E��һ�����������ɵĸ߷��ӻ�����Ľṹ��ʽΪ________________________________��
��5��X��F��ͬ���칹�壬��ͬʱ��������3�����������ܷ���ˮ�ⷴӦ���ڲ�������
���ܷ���������Ӧ����X���ܵĽṹ��ʽΪ_____________________________________��
���𰸡���1��2��1��ϩ����OH����CHO
��2��������Ӧ(��ȡ����Ӧ) ��CH3CH===C(CH3)COOCH2CH3
��3��  ��2Cu(OH)2��OH��
��2Cu(OH)2��OH��![]()
 ��Cu2O����3H2O
��Cu2O����3H2O
(ע���������������÷֣� �����ӵĵ������dz����һ������ɡ�)
�����ӵĵ������dz����һ������ɡ�)
��4��
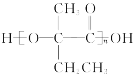 ��5�� HCOOCH2CH2CH=CH2��
��5�� HCOOCH2CH2CH=CH2��![]() ��
��![]()
��������
���������A����ˮ��Ӧ����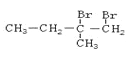 ����AΪ
����AΪ![]() ��BΪ
��BΪ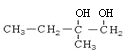 ��CΪ
��CΪ ��EΪ
��EΪ ����H�ķ���ʽ��֪��E������ȥ��Ӧ����F��CH3CH==C(CH3)COOH��G��F����������Ӧ����H��
����H�ķ���ʽ��֪��E������ȥ��Ӧ����F��CH3CH==C(CH3)COOH��G��F����������Ӧ����H��
��1��AΪ![]() ������Ϊ2��1��ϩ��CΪ
������Ϊ2��1��ϩ��CΪ �������ŵĽṹ��ʽΪ��OH����CHO��
�������ŵĽṹ��ʽΪ��OH����CHO��
��2��G��F�D��H�ķ�Ӧ����Ϊ������Ӧ��H�Ľṹ��ʽΪCH3CH===C(CH3)COOCH2CH3��
��3��C�D��D�����ӷ���ʽΪ
 ��2Cu(OH)2��OH��
��2Cu(OH)2��OH��![]()
 ��Cu2O����3H2O��
��Cu2O����3H2O��
��4��EΪ ����һ���������������۷�Ӧ���ɵĸ߷��ӻ�����Ľṹ��ʽΪ
����һ���������������۷�Ӧ���ɵĸ߷��ӻ�����Ľṹ��ʽΪ ��
��
��5��FΪCH3CH==C(CH3)COOH��X��F��ͬ���칹�壬��ͬʱ��������3�����������ܷ���ˮ�ⷴӦ��˵��������������ӦΪ����ij�����ڲ����������ܷ���������Ӧ��˵������ȩ������X���ܵĽṹ��ʽΪHCOOCH2CH2CH=CH2��![]() ��
��![]() ��
��

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�