��Ŀ����
����Ŀ��ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ��������ͼ��ʵ��װ�á�
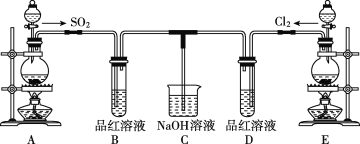
��1��ʵ������װ��E�Ʊ�Cl2���䷴Ӧ�Ļ�ѧ����ʽΪMnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O������6mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ____��
MnCl2+Cl2��+2H2O������6mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ____��
��2���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�B____��D____��
��ֹͣͨ�����ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ��B____��D____��
��3����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2��1��1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ���������������������������������ԭ��(�û�ѧ����ʽ��ʾ)��____��
���𰸡�3NA Ʒ����Һ��ɫ Ʒ����Һ��ɫ ��ɫ��Ʒ����Һ�ָֻ��ɺ�ɫ ���������� Cl2+SO2+2H2O=2HCl+H2SO4
��������
Aװ���Ʊ�SO2��Eװ��Ϊ�Ʊ�Cl2�����߶�����Ư���ԣ�����ʹƷ����Һ��ɫ����SO2��Ư���棬��ɫ�����Һ����ʱ�ָֻ�ԭ������ɫ����ˮ��Ư�ײ����棬���߶���NaOH��Ӧ������NaOH��Һ���ա�
��1����MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O�У�ÿ4molHCl���뷴Ӧ�����������Ȼ�����2mol�������������Ȼ���ռ���뷴Ӧ��
MnCl2+Cl2��+2H2O�У�ÿ4molHCl���뷴Ӧ�����������Ȼ�����2mol�������������Ȼ���ռ���뷴Ӧ��
50%��6molHCl�μӷ�Ӧ�����������Ȼ�����3mol��ת�Ƶĵ�����Ϊ3NA���ʴ�Ϊ��3NA��
��2����SO2����Ư���ԣ���������ͨ��Ʒ����Һ�У�Ʒ����ɫ��������ˮ��Ӧ���ɵĴ��������ǿ���ԣ��������ͨ��Ʒ����Һ��Ҳ��ʹƷ����ɫ���ʴ�Ϊ��Ʒ����Һ��ɫ��Ʒ����Һ��ɫ��
��SO2��Ư���棬��ɫ�����Һ����ʱ�ָֻ�ԭ������ɫ����ˮ��Ư�ײ����棬����ʱ��Ʒ����Һ���ָܻ���ɫ���ʴ�Ϊ����ɫ��Ʒ���ָֻ��ɺ�ɫ������������
��3���������н�ǿ�������ԣ�����������н�ǿ�Ļ�ԭ�ԣ���ˮ��Һ�����������ʵ���֮��1:1������Ӧ��Cl2+SO2+2H2O=2HCl+
H2SO4�������ﶼ��Ư���ԣ����SO2��Cl2�����ʵ���֮��1:1ͬʱͨ�뵽Ʒ����Һʱ��Ʒ����Һ������ɫ���ʴ�Ϊ��Cl2+SO2+
2H2O=2HCl+H2SO4��

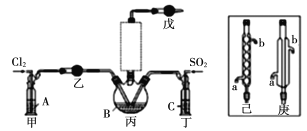
����Ŀ���������ó��ս��ì�ܼ�����Ҳ�����ǿ������й��뵼���ҵ���ڵ����㣬��˵�������Σ�Ϊʱδ�������ҳ����ڵIJ��㣬Ȼ����Ե�ȥ������⣬�����ð뵼���ҵ����չ׳�����������Ȼ�����(POCl3)��һ����Ҫ�Ļ���ԭ�ϣ��������뵼����Ӽ������άԭ�ϡ�һ�о�С����ʵ����ģ�ⷴӦ![]() �Ʊ�POCl3���ⶨ��Ʒ������
�Ʊ�POCl3���ⶨ��Ʒ������
���Ͽ�Ƭ��
���� | �۵㣯�� | �е㣯�� | ��Է������� | ���� |
PCl3 | -93.6 | 76.1 | 137.5 | ��ˮ����ˮ�⣬����O2��Ӧ |
POCl3 | 1.25 | 105.8 | 153.5 | ��ˮ����ˮ�⣬������PCl3 |
SOCl2 | -105 | 78.8 | 119 | ��ˮ����ˮ�⣬�����ֽ� |
��1����ѡ��Na2SO3������70��ŨH2SO4��ȡSO2����Ӧ�Ļ�ѧ����ʽ�ǣ�____________��
��2����ҺAΪ����ʳ��ˮ����װ����Ӧ��ʢװ���Լ�Ϊ__________(����P2O5��������ʯ��������ŨH2SO4��������ˮ����ͭ��)����Ӧװ��ͼ�������δ�������������ѡ��______(����������������)��
��3���ס���װ�õ����ó�����������ľ��������⣬����________________��
��4��ˮԡ����������ƿ�����Ʒ�Ӧ�¶���60��65�棬��ԭ����___________��
��5��ͨ��������·��ɲⶨ�����ᴿ��IJ�Ʒ��POCl3�ĺ�����ȷ��ȡ1��600g��Ʒ��ˮ��ƿ��ҡ������ȫˮ�⣬��ˮ��Һ���100 mL��Һ��ȡ10��00 mL����ƿ�У�����0��2000 mol��L-1��AgNO3��Һ20��00 mL(Ag++C1- AgC1��)���ټ�������������������ʹ�������л��︲�ǡ�����NH4Fe(SO4)2��ָʾ������0��1000 mol��L-1KSCN����Һ�ζ�������AgNO3���յ�(Ag++SCN- AgSCN��)����ƽ��ʵ�飬ƽ������KSCN����Һ10��00 mL��
�ٴﵽ�ζ��յ��������____________��
��POCl3����������Ϊ___________��
����֪��KSP(AgC1)=3
����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����8.2 g�������������������ʵ���Ʒ�����500 mL������Һ������ʱ����Ʒ�ɷ���________(����ĸ)������
A��С�ձ��С� ��B���ྻֽƬ�ϡ� ��C��������
��2���ζ������У��۾�Ӧע��________________���ζ�ʱ����0.2000molL-���������ζ�����Һ������ѡ��______��������ĸ����ָʾ����
A������ B��ʯ�� C����̪
��3���ζ�ʱ����0.200 0 mol��L��1���������ζ�������Һ�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����________ mol��L��1���ռ���Ʒ�Ĵ�����________��
�ζ����� | ������Һ�����mL�� | ������� | |
�ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
��һ�� | 10.00 | 0.40 | 20.50 |
�ڶ��� | 10.00 | 4.10 | 24.00 |
��4������ʵ�������Եζ��������ʲô�����������ƫ��������ƫ����������Ӱ������
��������ƿ�ô���Һ��ϴ��Ȼ�����10.00mL����Һ����ζ����______
������ƿδ����ͼ���10.00mL����Һ����ζ����______��