��Ŀ����
�������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ���㷺Ӧ���ڻ���ɱ��������������
��������ƾ�����Ʊ�
��ҵ������CaO2��8H2O����Ҫ�������£�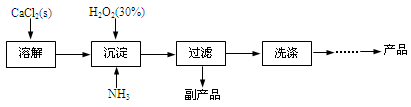
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2������ʱ���ñ�ˮ�����¶���10�����º�ͨ�������NH3�������ԭ��ֱ���
�� ���� ��
��������ƾ��庬���IJⶨ
ȷ��ȡ0.3000g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.0200 mol��L��1KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4����5 H2O2��6H+ ��2Mn2+��5O2����8H2O
��3���ζ��յ�۲쵽������Ϊ ��
��4�����ݱ�1���ݣ������Ʒ��CaO2��8H2O������������д��������̣�
| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.3000 | 1.02 | 24.04 |
| 2 | 0.3000 | 2.00 | 25.03 |
| 3 | 0.3000 | 0.20 | 23.24 |
18����12�֣�
��1��CaCl2+H2O2+2NH3+8H2O��CaO2��8H2O��+2NH4Cl ��2�֣�
��2�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ����ֹ��������ķֽ⣩��2�֣�
��ͨ�����NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣨��ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣬����߲�Ʒ�IJ��ʡ���˼��������֣���2�֣�
��3�����������һ��KMnO4����Һ����Һ����ɫ��dz��ɫ����30s����ɫ��2�֣�
��4��82.91%
5(CaO2��8H2O)��5H2O2��2KMnO4��1�֣�
n(CaO2��8H2O)�� n(KMnO4)
n(KMnO4)
�� ��0.0200 mol��L��1��23.03mL��10��3L��mL��1��1�֣�
��0.0200 mol��L��1��23.03mL��10��3L��mL��1��1�֣�
��1.151��10��3 mol��1�֣�
CaO2��8H2O����������Ϊ�� ��100%��82.91%��1�֣�
��100%��82.91%��1�֣�
����

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д��±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | �� | �� | |
��1���ܡ��ݡ������Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��2���ߡ��ࡢ�����ۺ������������ǿ������˳����_________________________��
��3���ɱ��Т٢�Ԫ�ص�ԭ�Ӱ�1��1��ɵĻ������ϡ��Һ�ױ����ֽ⣬ͨ��ʹ�õĴ���Ϊ������ţ�____________________��
a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4
��4����ͼ�� A��F���ɲ����ϱ���Ԫ����ɵĵ��ʻ������A��B��CΪ���ʣ�ת����ϵ���£�

I.��BΪ��ɫ���壬AΪԭ�Ӱ뾶��С��ԭ����ɵĵ��ʣ�CΪ˫ԭ�ӷ�����ɵĵ��ʣ�E��ʹƷ����Һ��ɫ��
��F�ĵ���ʽΪ ��
��ʵ������ʼ�μӷ�Ӧ��B��������ɵ�B������ȣ�����ʼ�μӷ�Ӧ��A��C�����ʵ���֮���� ��
II.��DΪ����ɫ���壬��ɫ��ӦΪ��ɫ�����C��Ԫ�ص�ԭ���������������ڲ��������2����
�����й���D��˵����ȷ���� ������ĸ����
a.����ˮ�������Ϸ�Ӧ
b.���������ԣ����л�ԭ��
c.�Ⱥ����Ӽ����ֺ��Ǽ��Թ��ۼ�
d.��һ�ּ���������
���ö��Ե缫��F�ı�����Һ���е�⣬��������Ӧʽ�� ��
abcd�����ֶ�����Ԫ�ء�a��b��dͬ���ڣ�c��dͬ����.aԭ�ӽṹʾ��ͼ ��b��c�γɻ�����ĵ���ʽΪ��ͼ
��b��c�γɻ�����ĵ���ʽΪ��ͼ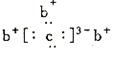 �����бȽ�����ȷ����
�����бȽ�����ȷ����
| A��ԭ�Ӱ뾶��a ��c��d | B���ǽ����ԣ�a ��b��d |
| C�����ʵ��۵㣺c�� a | D������������Ӧ��ˮ��������ԣ�c��d��a |
���и����л���������ʱȽϣ�����ȷ���� �� ��
| A�����ԣ�HClO4��HBrO4��HIO4 |
| B�����ԣ�NaOH��Mg(OH)2��Al(OH)3 |
| C���ǽ����ԣ�F��O��S |
| D���ȶ��ԣ�PH3��H2S��HCl |