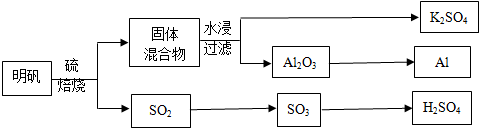
��Ŀ����
15��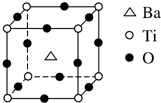 �Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺
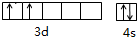
�Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺��1������${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti����ԭ�ӣ����ǻ���Ϊͬλ�أ�TiԪ����Ԫ�����ڱ��е�λ���ǵ������ڣ���IVB�壻��Ԫ�����ڱ�����������d��Ԫ�أ���̬ԭ�ӵļ۵��ӵĹ����ʾʽΪ
 ��
����2��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹ����ͼ��ʾ�����Ļ�ѧʽ��BaTiO3��
���� ��1����������ͬ����������ͬ��ͬ��Ԫ�صIJ�ͬԭ�ӻ�Ϊͬλ�أ�������Ԫ�������ڱ��е�ԭ�������Լ���ԭ�ӽṹ��ȷ��λ�á���̬ԭ�ӵĵ����Ų�ʽ��
��2�������з�̯�ĸ���ԭ�ӵ���Ŀ֮�ȼ��Ƿ����и�Ԫ��ԭ�ӵ���Ŀ���ݴ�д��ѧʽ��
��� �⣺��1��2248Ti��2250Ti����������ͬ����������ͬ������Ԫ�صIJ�ͬԭ�ӣ���Ϊͬλ�أ���Ԫ�������ڱ��е�ԭ������Ϊ22��λ�ڵ������ڵڡ�IVB�壬��d������̬ԭ�ӵĵ����Ų�ʽΪIs22s22p63S23p63d24s2����[Ar]3d24s2�������Լ۵��ӵĹ����ʾʽΪ ��
��
�ʴ�Ϊ��ͬλ�أ��ģ�IVB��d�� ��
��
��2�������з�̯�ĸ���ԭ�ӵ���Ŀ֮��Ba��Ti��O=1����8��$\frac{1}{8}$������12��$\frac{1}{4}$��=1��1��3���ʷ���ʽΪ��BaTiO3��
�ʴ�Ϊ��BaTiO3��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ�Ӻ�������Ų���֪ʶ�㣬���ؿ���֪ʶǨ�Ƽ��ռ������������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
5�������йؼ������˵���У�����ȷ���ǣ�������
| A�� | IA��Ԫ�ض��Ǽ����Ԫ�� | B�� | K�Ļ�ԭ��ǿ��Na | ||
| C�� | Na��Na+����ʱ����ʹ����ʻ�ɫ | D�� | Na�ڿ�����ȼ��ʱ����Na2O2 |
6��������ʵ����˵��Ԫ�ؼĽ�����һ����Ԫ���ҵĽ�����ǿ���ǣ�������
�ټ������ҵ�����Һ������ѧ��Ӧ
�ڳ����£���������Ũ������ҷ�Ӧ���ҵ���ȴ����
�ۼס�����Ԫ��ԭ�ӵ�������������ͬ���Ҽ�ԭ�Ӱ뾶С���ҵ�ԭ�Ӱ뾶
�ܼס���������������Ԫ��ԭ�ӵĵ��Ӳ�����ͬ���Ҽ�ԭ�Ӱ뾶С���ҵ�ԭ�Ӱ뾶��
�ټ������ҵ�����Һ������ѧ��Ӧ
�ڳ����£���������Ũ������ҷ�Ӧ���ҵ���ȴ����
�ۼס�����Ԫ��ԭ�ӵ�������������ͬ���Ҽ�ԭ�Ӱ뾶С���ҵ�ԭ�Ӱ뾶
�ܼס���������������Ԫ��ԭ�ӵĵ��Ӳ�����ͬ���Ҽ�ԭ�Ӱ뾶С���ҵ�ԭ�Ӱ뾶��
| A�� | ȫ�������� | B�� | ȫ���������� | C�� | �ڢۢ� | D�� | ���ٿ��� |
3������������������ǣ�������
| A�� | ��ѧ��Դ���Ǹ���ԭ��صĹ���ԭ����Ƶ� | |
| B�� | ��ӦA+B=C+DΪ���ȷ�Ӧ����A�������������ʾ��е����� | |
| C�� | ʯīת��Ϊ���ʯʱҪ���ȣ���˵��ʯī�Ƚ��ʯ�ȶ� | |
| D�� | ȼ�ϵ����һ�ָ�Ч�����������ͻ�ѧ��Դ |
10�����������ֲ�ͬ����������϶��γɵĻ��������NaOH��Һ��Ӧ�Ĺ��У�������
��-CH3����-OH����-C6H5����-CHO����-COOH��
��-CH3����-OH����-C6H5����-CHO����-COOH��
| A�� | 6�� | B�� | 5�� | C�� | 4�� | D�� | 3�� |
20�����й���ҩƷ��˵������ȷ���ǣ�������
| A�� | Ϊ��߳ɼ����������˶�Ա�����������з�����Ƽ� | |
| B�� | ��Ʒ��ָ���ڷ�ҽ��Ŀ�Ķ�����ʹ��ʹ�˲�������Ե�ҩƷ������ȡ������� | |
| C�� | ��˾ƥ�ֿ��Լ����ð�����ͷʹ�������ܴ������� | |
| D�� | ����ù��ǰһ��Ҫ����Ƥ������ |