��Ŀ����
6��������ʵ����˵��Ԫ�ؼĽ�����һ����Ԫ���ҵĽ�����ǿ���ǣ��������ټ������ҵ�����Һ������ѧ��Ӧ
�ڳ����£���������Ũ������ҷ�Ӧ���ҵ���ȴ����
�ۼס�����Ԫ��ԭ�ӵ�������������ͬ���Ҽ�ԭ�Ӱ뾶С���ҵ�ԭ�Ӱ뾶
�ܼס���������������Ԫ��ԭ�ӵĵ��Ӳ�����ͬ���Ҽ�ԭ�Ӱ뾶С���ҵ�ԭ�Ӱ뾶��
| A�� | ȫ�������� | B�� | ȫ���������� | C�� | �ڢۢ� | D�� | ���ٿ��� |
���� ��ͭ���Ȼ�����Һ������ѧ��Ӧ��
��ͭ��Ũ������ҷ�Ӧ���������ܣ�
������Ƶ��������1�����ӣ������ԭ�Ӱ뾶С���Ƶ�ԭ�Ӱ뾶��
���ƺ�þ���Ӳ�����ͬ��þ��ԭ�Ӱ뾶С���ƣ�
��� �⣺��ͭ���Ȼ�����Һ������ѧ��Ӧ��������ͭ���ã��ʴ���
��ͭ��Ũ������ҷ�Ӧ���������ܣ�������ͭ���ã��ʴ���
������Ƶ��������1�����ӣ������ԭ�Ӱ뾶С���Ƶ�ԭ�Ӱ뾶�����Ƿǽ��������ǽ������ʴ���
���ƺ�þ���Ӳ�����ͬ��þ��ԭ�Ӱ뾶С���ƣ����Ʊ�þ�Ľ�����ǿ���ʴ���
��ѡB��
���� ���⿼������Ļ����Ե����⣬ѧ��ֻҪѧ��Ӧ����ѧ֪ʶ���оٷ����Ϳ���Ѹ�ٽ��⣬�Ƚ����ף�

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
16��ij�о�С��Ϊ��̽��������ˮ�������ķ�Ӧ�IJ������������ʵ�飺����ͼ1��ʾʵ��װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��ʯ�������²���ˮ������Ӧ����

��1����μ���װ�õ������ԣ���C��������ĩ�˽���ˮ���У�����A��Բ����ƿ������ĩ�˳������ݣ�ֹͣ���Ⱥ�ĩ�˳���һ��ˮ��
��2��д������ˮ������Ӧ�Ļ�ѧ����ʽ3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2
��3����֤�����������Ԫ�صļ�̬
��ѡʵ���������Լ����ձ����Թܡ���������ҩ�ס��ιܡ��ƾ��ơ��ԹܼУ�1mol/L CuSO4��3mol/L H2SO4��3mol/L HNO3��30%H2O2��0.01mol/L KMnO4��20%KSCN������ˮ��
�ڴ���ϰ��±��ĸ�ʽд��ʵ�鲽�衢Ԥ����������ۣ�
����1�м�������1mol/L CuSO4��Һ�������dz�ȥ��Ӧ������п���δ��Ӧ������ۣ�����Ӱ�����ʵ��Ľ��������
��4�������������Ԫ�ص����������IJⶨ�ɲ���ͼ2�����̣����в����������Һ�м���п������ɫ�պ���ʧ���������0.010mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һƽ��Ϊv mL������֪5Fe2++MnO4-+8H+�T5Fe3++Mn2++4H2O�����м���п�۵�Ŀ���ǽ�Fe3+��ԭΪFe2+��ʵ����ag��Ʒ�й��������Ԫ�ص���������Ϊ$\frac{250��5��56g/mol��0.010mol/L��V��1{0}^{-3}L}{25a}$��100%_��ֻ�м������ʽ����

��1����μ���װ�õ������ԣ���C��������ĩ�˽���ˮ���У�����A��Բ����ƿ������ĩ�˳������ݣ�ֹͣ���Ⱥ�ĩ�˳���һ��ˮ��
��2��д������ˮ������Ӧ�Ļ�ѧ����ʽ3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2
��3����֤�����������Ԫ�صļ�̬
��ѡʵ���������Լ����ձ����Թܡ���������ҩ�ס��ιܡ��ƾ��ơ��ԹܼУ�1mol/L CuSO4��3mol/L H2SO4��3mol/L HNO3��30%H2O2��0.01mol/L KMnO4��20%KSCN������ˮ��
�ڴ���ϰ��±��ĸ�ʽд��ʵ�鲽�衢Ԥ����������ۣ�
| ʵ�鲽�� | Ԥ����������� | |
| ����1 | ȡ��Ӧ�����Ĺ���ag���Թ��У�����������1mol/L CuSO4��Һ�����������Һ���롢ϴ�Ӻ���������еμ�������3mol/LH2SO4�����ܽ⣬���˺���Һ���250mL��Һ�����ã� | - |
| ����2 | ȡ��������1����Һ���Թ��У��μ�1��2��20%KSCN | ��Һ���ɫ���������ﺬ+3���� |
| ����3 | ȡ��������1����Һ���Թ��У��μ�1��2��0.01mol/LKMnO4 | ��Һ��ɫ��ȥ���������ﺬ+2���� |
��4�������������Ԫ�ص����������IJⶨ�ɲ���ͼ2�����̣����в����������Һ�м���п������ɫ�պ���ʧ���������0.010mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һƽ��Ϊv mL������֪5Fe2++MnO4-+8H+�T5Fe3++Mn2++4H2O�����м���п�۵�Ŀ���ǽ�Fe3+��ԭΪFe2+��ʵ����ag��Ʒ�й��������Ԫ�ص���������Ϊ$\frac{250��5��56g/mol��0.010mol/L��V��1{0}^{-3}L}{25a}$��100%_��ֻ�м������ʽ����
17������ʱ��9��25��21ʱ10��04�룬�ҹ�������ҵ��ӭ��һ����ʷ��ʱ�̣��ҹ��������Ƶ������ߺ����˷ɴ��ھ�Ȫ���Ƿ������ķ������գ���28������ص������ĵ������ۡ��ߺŻ㼯�˴������¿Ƽ����������ij����ƽ���Ϊ����������ƫ�����£�C2H8N2����5.00g C2H8N2��ȫȼ�տɷų�212.5kJ����������������ȷ���ǣ�������
| A�� | ȼ�ղ�һ���������μӣ�Ҳ��һ���Ƿ��ȷ�Ӧ | |
| B�� | �����������ĺ�ɫ�����ǽ�������ɫ��Ӧ������ | |
| C�� | ���ȼ��ȼ����Ҫ�ǽ���ѧ��ת��Ϊ���ܺ��ܣ����ܶԻ���������Ⱦ | |
| D�� | ƫ������ȼ�յ��Ȼ�ѧ����ʽ�ǣ�C2H8N2��g��+2N2O4��g���T2N2��g��+2CO2��g��+4H2O��g������H=-2550kJ/mol |
14��ij�л�����Ʒ3.1g��ȫȼ�գ����ۺ��ֱ�����ϣ�ȼ�պ�Ļ����ͨ������ij���ʯ��ˮ��ʯ��ˮ������7.1g�������˺�õ�10g���������л�����Ʒ�����ǣ�������
���Ҷ��� ���Ҵ� ����ȩ �ܼ״��ͱ������Ļ���
���Ҷ��� ���Ҵ� ����ȩ �ܼ״��ͱ������Ļ���
| A�� | �٢� | B�� | �� | C�� | �٢� | D�� | �� |
1�����������֣�NASA������ŵ��ʿ�ҵ���һ�ֱȶ�����̼��Ч104���ġ������������塱--ȫ�����飨C3F8������������䡰���һ����ǡ�ʹ���Ϊ�ڶ�������ļƻ����й�ȫ�������˵����ȷ���ǣ�������
| A�� | ����������̼ԭ�ӿ��ܴ���ͬһֱ���� | |
| B�� | ȫ������ĵ���ʽΪ��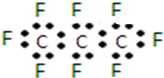 | |
| C�� | ��ͬѹǿ�£��е㣺C3F8��C3H8 | |
| D�� | ȫ����������Ǽ��м��Լ����зǼ��Լ��ļ��Է��� |
11������ʽΪ��C7H8O3N2���л����з��ϣ�����������ֱ�����ڱ����ϲ��ʶ�λ��ͬ���칹�����У�������
| A�� | 12�� | B�� | 10�� | C�� | 6�� | D�� | 4�� |
18�������й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������

| A�� | ��ͼ1��ʾװ�ó�ȥCl2�к��е�����HCl | |
| B�� | ��ͼ2��ʾװ����MgCl2��Һ�Ʊ���ˮMgCl2���� | |
| C�� | ��ͼ3��ʾװ����ȡ����CO2���� | |
| D�� | ��ͼ4��ʾװ�ó�ȥ�屽�л��е�����Br2 |
16�����������и������ӵ���Һ���ܹ�ʵ�ֵ��ǣ�������
| A�� | Cu2+��Br-��HCO3-��AlO2- | B�� | Fe3+��K+��SCN-��SO42- | ||
| C�� | Fe2+��NO3-��HSO3-��Ag+ | D�� | Na+��AlO2-��K+��C6H5O- |
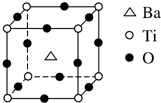 �Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺
�Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺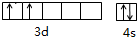 ��
��