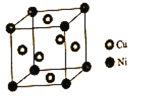
��Ŀ����
����Ŀ�����ܺϽ����Բ�Ϊ�����ܶ�Ԫ�Ͻ��ڸ����£������ܿ������ܣ��������Ϊ�������������ܺϽ���Լ�ǿ�����ȶ��Խϸߣ��ͻ�ѧ��ʴ�Ժܺã���Ҫ���ں��캽���DZ��������ӱ����ſعܵȡ�
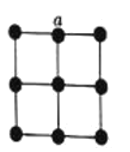
(1)��̬��ԭ�ӵļ۵����Ų�ͼΪ___________��
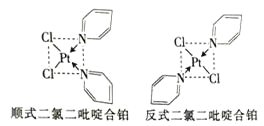
(2)���ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ�������˳ʽ�ͷ�ʽ����ͬ���칹��![]() ��ͼ
��ͼ![]() ����ѧ�о�������˳ʽ���Ӿ��п������ԡ�
����ѧ�о�������˳ʽ���Ӿ��п������ԡ�
����ष����Ǵ����ƽ�����壬�ṹ��ʽ��ͼ��ʾ����ष�����Nԭ�ӵ��ӻ���ʽ��____�������еĴ��������÷���![]() ��ʾ������m��ʾ�����γɴ�������ԭ�Ӹ�����n��ʾ�����γɴ������ĵ��Ӹ�����������еĴ�����Ӧ��ʾΪ_____��
��ʾ������m��ʾ�����γɴ�������ԭ�Ӹ�����n��ʾ�����γɴ������ĵ��Ӹ�����������еĴ�����Ӧ��ʾΪ_____��
�ڶ��ȶ���ऺϲ�������������C��N��Cl����Ԫ�صĵ�һ�������ɴ�С��˳����_____��
�۶��ȶ���ऺϲ������д��ڵ�������������_________![]() ����ĸ
����ĸ![]() ��
��
![]() ���Ӽ�
���Ӽ� ![]() ��λ��
��� ![]() ������
������ ![]() �Ǽ��Լ�
�Ǽ��Լ� ![]() ���
��� ![]() ���Լ�
���Լ�
�ܷ�ʽ���ȶ���ऺϲ�������_________![]() �������Է����������Ǽ��Է�����
�������Է����������Ǽ��Է�����![]() ��
��
�ݶ��ȶ���ऺϲ�������ƽ��ṹ��Pt2+����λ����4�����������ӻ���ʽ������sp3����������______________��
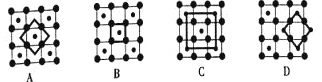
(3)�������Ͽ�ѧ����ʵ����һ������С�鷢������5K�³��ֳ����Եľ���CoO2���þ�����в�״�ṹ![]() ��ͼ��ʾ��С���ʾCoԭ�ӣ������ʾOԭ��
��ͼ��ʾ��С���ʾCoԭ�ӣ������ʾOԭ��![]() ��ͼ���ô��������ظ��ṹ��Ԫʾ��ͼ��������CoO2�Ļ�ѧ��ɵ���__________
��ͼ���ô��������ظ��ṹ��Ԫʾ��ͼ��������CoO2�Ļ�ѧ��ɵ���__________![]() ����ĸ
����ĸ![]() ��
��
(4)�����������У���ԭ�ӵ���λ��Ϊ12��������������x��y��z���ͶӰͼ��ͼ��ʾ�������������ܶ�Ϊd g
���𰸡�![]() sp2
sp2 ![]() N>Cl>C bdf �Ǽ��Է��� ����ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ B
N>Cl>C bdf �Ǽ��Է��� ����ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ B ![]() ��
��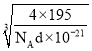
��������
(1)Co���ڵ������ڵ�VIII�壬�۵����Ų�ʽΪ3d74s2��
(2)������वĽṹ��ʽ���Կ�����Nԭ���γ�2��������N�ϻ���1�Թµ��Ӷԣ���ष�����5��̼ԭ��Ҳ��ȡsp2�ӻ���
��ͬ��������Ԫ����ԭ�����������һ�����ܳ��������ƣ�NԪ��ԭ��2p���Ϊ������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�
�۶��ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ��������ڷ��Ӿ��壻
�� Cl-����ा�����Pt2+�ʶԳƽṹ��������������������غϣ�
�ݶ��ȶ���ऺϲ������У�Pt2+����λ����4�����������ӻ���ʽ������sp3��
(3)CoO2���ظ��ṹ��Ԫʾ��ͼ��Co��Oԭ����Ŀ֮��ӦΪ1:2����ͼ���֪��
A��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��2��
=1��2��
B��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��1��
=1��1��
C��Co��Oԭ����Ŀ֮��Ϊ (1+4��![]() )��4=1��2��
)��4=1��2��
D��Co��Oԭ����Ŀ֮��Ϊ4��![]() ��4��
��4��![]() =1��2��
=1��2��
(4)���ݾ����ṹ����=![]() ���㡣
���㡣
(1)Co���ڵ������ڵ�VIII�壬�۵����Ų�ʽΪ3d74s2���۵����Ų�ͼΪ��![]() ��
��
(2)������वĽṹ��ʽ���Կ�����Nԭ���γ�2��������N�ϻ���1�Թµ��Ӷԣ���ष����е�ԭ�ӵ��ӻ���ʽ��sp2�ӻ�����ष�����5��̼ԭ��Ҳ��ȡsp2�ӻ���ÿ��Cԭ�ӵ�δ�����ӻ���2p����ϵ�1�����Ӻ�Nԭ�ӵ�δ�����ӻ���2p����ϵ�1�������γɴ������������γɴ�������ԭ����Ϊ6��������Ϊ6������еĴ�������ʾΪ![]() ��
��
��ͬ��������Ԫ����ԭ�����������һ�����ܳ��������ƣ�NԪ��ԭ��2p���Ϊ������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�N��ԭ�Ӱ뾶С��Cl�ģ���2p���Ϊ������ȶ��ṹ����һ�����ܴ���Cl�ģ�Cl��̼��ȣ�ʧȥ���ӵ�������������Cl�ĵ�һ�����ܴʵ�һ������N>Cl>C��
�۶��ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ��������ڷ��Ӿ��壬����֮����ڷ��»�����Pt2+��Cl-������γ���λ����û�����Ӽ��������̼ԭ��֮���γɷǼ��Լ�����ͬԭ��֮���γɼ��Լ���C-H������ԭ�Ӳ����γ������Ҳû�н��������ʴ�Ϊ��bdf��
�ܷ�ʽ���ȶ���ऺϲ�������Cl-����ा�����Pt2+�ʶԳƽṹ��������������������غϣ����ڷǼ��Է��ӣ�
�ݶ��ȶ���ऺϲ������У�Pt2+����λ����4�����������ӻ���ʽ������sp3������������ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ��
(3)CoO2���ظ��ṹ��Ԫʾ��ͼ��Co��Oԭ����Ŀ֮��ӦΪ1:2����ͼ���֪��
A��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��2�����ϣ�
=1��2�����ϣ�
B��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��1�������ϣ�
=1��1�������ϣ�
C��Co��Oԭ����Ŀ֮��Ϊ (1+4��![]() )��4=1��2�����ϣ�
)��4=1��2�����ϣ�
D��Co��Oԭ����Ŀ֮��Ϊ4��![]() ��4��
��4��![]() =1��2�����ϣ�
=1��2�����ϣ�
��ѡB��
(4)�����������У���ԭ�ӵ���λ��Ϊ12��Ϊ���������������x��y��z���ͶӰͼ����֪��Ϊ�����������ܶѻ���Ptԭ�Ӵ��ڶ��㡢���ģ�������Ptԭ����Ŀ=8��![]() +6��
+6��![]() =4����
=4����![]() =d��a3��10-21�����
=d��a3��10-21�����![]() ��
�� ��
��

����Ŀ����������Ȼ�ѧѭ����ͨ������ѭ��������ѭ����ȡ��������������ѭ���������Ȼ�ѧѭ����ԭ������ͼ��ʾ��

��1���������.�����ȷֽ⡱�ں����ܱ������н��У���ø����ʵ����ʵ����������¶ȵĹ�ϵ����ͼ��ʾ������650��1200��䷢������Ҫ��Ӧ�ķ���ʽΪ____��
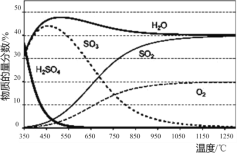
��2���������.���������������ӷ���ʽΪ____��HI��ǿ�ᣩ��
��3�������ķ�ӦΪ2HI(g) ![]() H2(g) + I2(g) ��
H2(g) + I2(g) ��
�����ں��º����ܱ������н��и÷�Ӧ����˵���Ѵﵽƽ��״̬����___������ţ���
a���������������ѹǿ������ʱ����仯
b��n(HI)��n(H2)��n(I2)=2��1��1
c����Ӧ���ʣ�v(H2)��=v(H2)��
d��I2(g)Ũ�Ȳ�����ʱ��ı仯���仯
����֪���ѣ������ɣ�1mol��ѧ�����գ���ų�����������Ϊ���ܡ���ؼ����������£�
��ѧ�� | H��I | H��H | I��I |
����/kJ��mol��1 | 298.7 | 436.0 | 152.7 |
��÷�Ӧ��![]() HΪ____kJ��mol��1��
HΪ____kJ��mol��1��
��4����������ѭ�����в��������ͼװ�ô��漴Ϊ������ѭ������
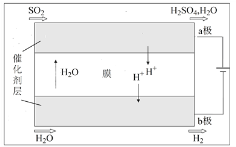
�����йظ�װ�õ����˵����ȷ����____������ţ���
a����ѧ��ת��Ϊ����
b�������ɼӿ�缫�ϵ��ӵ�ת��
c����Ӧ���ܷ���ʽΪSO2+2H2O ![]() H2+H2SO4
H2+H2SO4
d��ÿ����1molH2����·�������ĵ�����ԼΪ6.02��1023