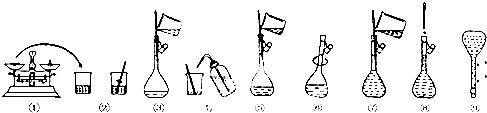��Ŀ����
3����1���ý�������ȡ�������ƵĻ�ѧ����ʽ��2Na+O2$\frac{\underline{\;����\;}}{\;}$Na2O2�������Ƿ�����ɫ���棬���ɵ���ɫ���壮��2���ý�������ȡNa2Oͨ�������·���2NaNO2+6Na�T4Na2O+N2��������������������ȼ�ղ��ô˷���ԭ�����Ƶ������Ƶ�ͬʱ����N2��N2��Ϊ����������ֹNa2O��һ��������ΪNa2O2��
���� ��1���ƺ������ڵ�ȼ����������·�����ɫ�������ɵ���ɫ����������ƣ�
��2���ƺ�������Ӧ���������ƣ�������������ȼ�����ɹ������ƶ��ò��������ƣ�
��� �⣺��1���ƺ������ڵ�ȼ����������·�Ӧ���ɹ������ƣ���Ӧ�ķ���ʽΪ��2Na+O2$\frac{\underline{\;����\;}}{\;}$Na2O2��������ɫ���棬���ɵ���ɫ���壻
�ʴ�Ϊ��2Na+O2$\frac{\underline{\;����\;}}{\;}$Na2O2��������ɫ���棬���ɵ���ɫ���壻
��2������������ȼ�ս��õ�Na2O2�����������������Ƶ������Ƶ�ͬʱ����N2��N2��Ϊ����������ֹNa2O��һ��������ΪNa2O2�����Բ��ô˷���ȡ�����ƣ�
�ʴ�Ϊ���Ƶ������Ƶ�ͬʱ����N2��N2��Ϊ����������ֹNa2O��һ��������ΪNa2O2��
���� ������Ҫ�����ơ��������Ƶ����ʣ������ڻ���֪ʶ�Ŀ����Ӧ�ã�����������ѧ�������ÿ�ѧ�������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
11�����и��黥Ϊͬ���칹����ǣ�������
| A�� | O2��O3 | B�� | ${\;}_{1}^{1}$H��${\;}_{1}^{2}$H | ||
| C�� | CH3CH3��CH3CH2 CH3 | D�� | CH3CH2CH2CH3��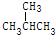 |
8�������Ȼ�ѧ����ʽ�����ӷ���ʽ�У���ȷ���ǣ�������
| A�� | �������������������NaOH��Һ��Al2O3+2OH-+3H2O�T2Al��OH��3�� | |
| B�� | ̼�����Ƶ�ˮ�⣺HCO3-+H2O?H3O++CO32- | |
| C�� | 500�桢30MPa�£���0.5mol N2��1.5mol H2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-38.6KJ/mol | |
| D�� | ����ı�ȼ����Ϊ-890.3kJ•mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g���TCO2��g��+2H2O��1����H=-890.3kJ•mol-1 |
13���±��г��ˢ١�������Ԫ����Ԫ�����ڱ��е�λ�ã�
��ش��������⣺
��1���۵�Ԫ�ط�����Mg��
��2���ڡ��ۡ�������Ԫ����Ƚϣ���������ǿ����Na ����дԪ�ط��ţ���
��3��Ԫ�آݺ͢��⻯���У��ȶ��Խ�ǿ����HF�����⻯��ķ���ʽ����
��4���ܵ�����������Ӧ��ˮ����Ļ�ѧʽΪAl��OH��3��
��5������Ԫ�آ۵�ԭ�ӽṹʾ��ͼ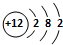 ��
��
��6��Ԫ�آ۵ĵ��������ᷴӦ�����ӷ���ʽΪMg+2H+=Mg2++H2����
��7��Ԫ�آ��γɵ����磺̼���ƣ���̼�����к���̼����������ʱ����ȥ�����ʵļ����Ǽ��ȣ����ȡ�����ȡ���������
| �� ���� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� |
��1���۵�Ԫ�ط�����Mg��
��2���ڡ��ۡ�������Ԫ����Ƚϣ���������ǿ����Na ����дԪ�ط��ţ���
��3��Ԫ�آݺ͢��⻯���У��ȶ��Խ�ǿ����HF�����⻯��ķ���ʽ����
��4���ܵ�����������Ӧ��ˮ����Ļ�ѧʽΪAl��OH��3��
��5������Ԫ�آ۵�ԭ�ӽṹʾ��ͼ
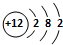 ��
����6��Ԫ�آ۵ĵ��������ᷴӦ�����ӷ���ʽΪMg+2H+=Mg2++H2����
��7��Ԫ�آ��γɵ����磺̼���ƣ���̼�����к���̼����������ʱ����ȥ�����ʵļ����Ǽ��ȣ����ȡ�����ȡ���������
 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺