��Ŀ����
15����ͼΪ����250mL 0.2mol/L Na2CO3��Һ��ʾ��ͼ��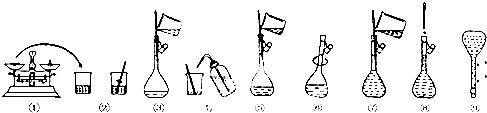
�ش��������⣺
��1�����гƵ�Na2CO35.3g��ѡȡ����ƿ���250mL����ƿ��
��2������ƿʹ��ǰӦ���м���Ƿ�©ˮ������
��3�������������������������ҺŨ���к�Ӱ�죿���ƫ�ߡ���ƫ�͡�����Ӱ�족��
A��ijͬѧ�ڵڢಽ�۲�Һ��ʱ����ƫ�ߣ�
B��û�н��в�������ܺ͢�ƫ�ͣ�
C���ڵڢݲ�����������Һ����������ƿ��ƫ�ͣ�
D��δ����ȴ���Ƚ���Һע������ƿ�ж���ƫ�ߣ�
E��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮƫ�ͣ�
���� ��1���������ʵ�����m=nM=cvM���㣻����ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ���ݴ�ѡ��
��2������ƿǰʹ��ǰ����Ƿ�©ˮ��
��3������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1��0.2mol•L-1Na2CO3��Һ250mL��ҪNa2CO3������Ϊ��0.25L��0.2mol/L��106g/mol=5.3g������ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ��������250mL��Һ��Ӧѡ��250mL����ƿ���ʴ�Ϊ��5.3��250mL����ƿ��
��2������ƿ��ʹ��ǰ����Ƿ�©ˮ���ʴ�Ϊ������Ƿ�©ˮ��
��3��A��ijͬѧ�ڵڢಽ�۲�Һ��ʱ���ӣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
B��û�н��в�������ܺ͢ݣ��ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
C���ڵڢݲ�����������Һ����������ƿ�⣬�ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
D��δ����ȴ���Ƚ���Һע������ƿ�ж��ݣ�����Һ��ȴ����Һ���ƫС����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
E��ҡ�Ⱥ���Һ����ڿ̶����������ģ��ټ�ˮ����ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

| ������̼������Һ��� | �������Ȼ�����Һ��� | ������̼��������Һ��� | CO2ͨ��CaCl2��Һ |
| A | B | C | D |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | $\frac{1000{��}_{1}{��}_{2}��}{182.5}$mol/L | B�� | $\frac{1000{��}_{1}{��}_{2}��}{{��}_{1}+4}$mol/L | ||
| C�� | $\frac{1000{��}_{1}{��}_{2}��}{36.5��{��}_{1}+4��}$mol/L | D�� | $\frac{1000{��}_{1}��}{182.5}$mol/L |
| A�� | ��ˮ�͵⻯�ط�Ӧ��Cl2+I-�TCl-+I2 | |
| B�� | ̼��������ᷴӦ��CO32-+2H+�TCO2��+H2O | |
| C�� | ������ˮ��Ӧ��Cl2+H2O�T2H++Cl-+ClO- | |
| D�� | ��ⱥ��ʳ��ˮ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$H2��+Cl2��+2OH- |
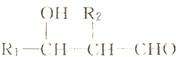
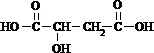 +2NaHCO3��
+2NaHCO3��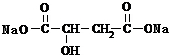 +2CO2��+2H2O��
+2CO2��+2H2O�� ��
�� ��
��