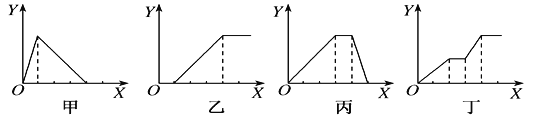��Ŀ����
����Ŀ�����������л�����������ա�
�� C2H4 �� C2H2 �� ![]() ��
��![]()
�� ![]() ��
��![]() ��
��![]() ��
��  �ᷴ��2����ϩ
�ᷴ��2����ϩ
��1����~���У����ڱ���ͬϵ�����____������ţ���ͬ����
��2���ڵĵ���ʽΪ___________��
��3���ܵ�ϵͳ����Ϊ ___________��
��4����Ľṹ��ʽΪ __________��
��5���۱�����KMnO4��Һ�������л�����Ľṹ��ʽΪ_____��__________��
��6�����ں˴Ź�����������_______��壻
��7���١� �ۡ� ��ķе��ɸߵ��͵�˳����____________��
��8��д���ݵĺ��б�������ݲ�ͬ����һ��ͬ���칹��Ľṹ��ʽ________________��
���𰸡��� ![]() 2��5-����-2��4-����ϩ
2��5-����-2��4-����ϩ 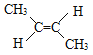
 CH3COOH 4 ��> ��>��
CH3COOH 4 ��> ��>�� 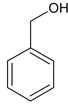 ��
��
��������
��1������ͬϵ���к���1���������������������ɸ�CH2�ṹ��
��2����Ϊ��Ȳ������̼̼������
��3���ܵĺ���̼̼˫����������6��̼ԭ�ӣ���Ϊ�Գƽṹ��˫���ڵڶ����ĸ�̼ԭ���ϣ�����2��5��̼ԭ���ϣ�
��4���ᷴ��2����ϩ��2������̼̼˫������ࣻ
��5������KMnO4��Һ������C=C����
��6���� ��3�����ϵ���ԭ����ͬ����������3����ԭ�ӣ�
��3�����ϵ���ԭ����ͬ����������3����ԭ�ӣ�
��7���� C2H4�� ![]() �ᷴ��2����ϩΪ���Ӿ��壬�е�ĸߵ���������йأ�������Խ�е�Խ�ߣ�
�ᷴ��2����ϩΪ���Ӿ��壬�е�ĸߵ���������йأ�������Խ�е�Խ�ߣ�
��8����![]() ��ͬ���칹���к��б����������ͬ������Ϊ�����ѣ�
��ͬ���칹���к��б����������ͬ������Ϊ�����ѣ�
��1������ͬϵ���к���1���������������������ɸ�CH2�ṹ��Ϊ�ࣻ
��2����Ϊ��Ȳ������̼̼����������ʽΪ![]() ��
��
��3���ܵĺ���̼̼˫����������6��̼ԭ�ӣ���Ϊ�Գƽṹ��˫���ڵ�2��4��̼ԭ���ϣ����ڵ�2��5��̼ԭ���ϣ�ϵͳ����Ϊ2��5-����-2��4-����ϩ��
��4���ᷴ��2����ϩ��2������̼̼˫������࣬�ṹ��ʽΪ ��
��
��5������KMnO4��Һ������C=C��������Ϊ  ��CH3COOH��
��CH3COOH��
��6���� ��3�����ϵ���ԭ����ͬ����������3����ԭ�ӣ���˴Ź���������4����ֵ��
��3�����ϵ���ԭ����ͬ����������3����ԭ�ӣ���˴Ź���������4����ֵ��
��7���� C2H4�� ![]() �ᷴ��2����ϩΪ���Ӿ��壬�е�ĸߵ�����Է��������йأ���Է�������Խ�е�Խ�ߣ��е��ɸߵ��͵�˳���Ǣ�> ��>�٣�
�ᷴ��2����ϩΪ���Ӿ��壬�е�ĸߵ�����Է��������йأ���Է�������Խ�е�Խ�ߣ��е��ɸߵ��͵�˳���Ǣ�> ��>�٣�
��8����![]() ��ͬ���칹���к��б�������������䲻ͬ���������Ϊ�����ѣ��ṹ��ʽΪ
��ͬ���칹���к��б�������������䲻ͬ���������Ϊ�����ѣ��ṹ��ʽΪ![]() ��
��![]() ��
��

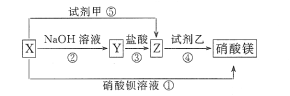
����Ŀ������п��ŨH2SO4����ڼ����·�Ӧ���ɻ�SO2��H2�Ļ�����壻п��ϡ���ᷴӦֻ��H2���ɡ���֪��Zn+2H2SO4(Ũ)![]() ZnSO4+2H2O+SO2�������м������о�С��ֱ�ʵ��̽����
ZnSO4+2H2O+SO2�������м������о�С��ֱ�ʵ��̽����
��1�����о�С�鰴��ͼʵ����֤п��Ũ���ᷴӦ��������SO2��H2��ȡ������Zn����b�У���a�м���100mL18.5mol��L��1��Ũ���ᣬ����һ��ʱ�䷴Ӧ��Zn��ȫ�ܽ⡣

����д�������ƣ�a___________��b___________��
���о�С����Ϊ�����ܲ��������������ǣ�_____________________��
��װ��D�м�����Լ���__________��
��U��G������Ϊ__________��
����ͬѧ��ΪA��B��Ӧ����ͼ�еļ�װ�ã���װ�õ�����Ϊ__________��
��֤����Ӧ����SO2��H2��ʵ��������______________________________��
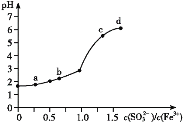
��2�����о�С��Ϊ��̽��п��ϡ���ᷴӦ�����е����ʼ������ı仯����������ʵ�飬����Ӱ�췴Ӧ���ʵ����ء�

ʵ��ʱ���ӶϿ�K��ʼ��ÿ���1���ӣ�����Ͽ���պ�K������������ÿ1�����ڴ�a��������ˮ�������õ���ˮ�������±���ʾ��
1����ˮ�������Ͽ�K�� | 34 | 59 | 86 | 117 | �� | 102 |
1����ˮ�������պ�K�� | 58 | 81 | 112 | 139 | �� | 78 |
������Ӧ�����е�ˮ��������ش�
�� ��ˮ����58��34��81��59��˵���ڷ�Ӧ���ڣ��պ�Kʱ�ȶϿ�Kʱ�ķ�Ӧ���ʿ죬��Ҫԭ����________��
�� ��ˮ����102��78��˵���ڷ�Ӧ���ڣ��Ͽ�Kʱ�ķ�Ӧ���ʿ��ڱպ�Kʱ�ķ�Ӧ���ʣ���Ҫԭ����______��
����Ŀ��2009��10��1�գ��ҹ��ɹ��ٰ������ʮ���ı���������ı���ʽ��9���綯�����϶�����������Դ���������࣬չʾ���ۺϹ����������Ƽ���չˮƽ��ͬʱҲ˵����Դ��ȱ������������ٵ��ش����⡣�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ�� CO(g) �� 2H2(g)![]() CH3OH(g) ��H1
CH3OH(g) ��H1
��Ӧ�� CO2(g) �� 3H2(g)![]() CH3OH(g) + H2O(g) ��H2
CH3OH(g) + H2O(g) ��H2
��������Ӧ������ԭ�Ӿ�����ԭ����� _____����������������������
���±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
�¶� | 250�� | 300�� | 350�� |
K | 2.041 | 0.270 | 0.012 |
�ɱ��������ж���H1 0 ������������������������������
��ij�¶��£���2 mol CO��6 mol H2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)�� 0.2 mol/L����CO��ת����Ϊ ����ʱ���¶�Ϊ �����ϱ���ѡ��
��2����֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(g) ��H1����1275.6 kJ/mol
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H2����566.0 kJ/mol
�� H2O(g) �� H2O(l) ��H3����44.0 kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ����
�������ͼ��ʾ�ĵ��װ�á��õ�������ĵ缫��ӦΪ ��

�ڹ���һ��ʱ������Һ��pH��С���õ���ܷ�Ӧ�Ļ�ѧ����ʽΪ
��