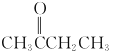��Ŀ����
����Ŀ����ü���������ֳ������£����ַ�������������⡱��������֮Ӱ�������У���ơ������⡱��Ϊ��üʤ��֮һ���������������и��������������µĺ�ҹ�ɼ�������ӫ��������
��1����ӫ�⡱��Ҫ�ɷ��� PH3��좣�����ṹʽΪ __________ �������й� PH3 ��˵���������___________ ��
a.PH3 �����Ǽ��Է���
b.PH3 �����ȶ��Ե��� NH3 ���ӣ���Ϊ N-H �����ܸ�
c.һ�� PH3 �����У�P ԭ�Ӻ�����һ�Թµ��Ӷ�
d.PH3 �е���� NH3 �е㣬��Ϊ P-H �����ܵ�
��2��PH3 �ķе�� NH3______��ߡ��͡��� NH3 ��ˮ��Һ����_____PH3 ��ˮ��Һ���ԣ�����ڡ���С�ڡ������Ȼ��l��PH4C1����Ӧ����좵����ӷ���ʽΪ _______________________ ��
��3��PH3 ��һ�ֻ�ԭ�����仹ԭ������ NH3 ǿ��ͨ��������ܴ�Cu2+��Ag+��Hg2+������Һ�л�ԭ�������� ������������Ϊ�������̬��PH3 �� CuSO4 ��Һ��Ӧ�Ļ�ѧ����ʽΪ ______________��
��4����ӫ�⡱������ԭ����Ca3P2 �ڳ�ʪ�Ŀ����о��ҷ�Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ__________________��
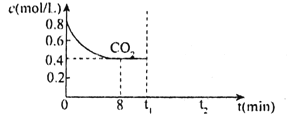
��5��PH3 �ж������������� Cu2+��Pd2+Һ���ѳ� PH3��PH3+2O2![]() H3PO4������������ͬʱ�� �ܽ�����Һ��O2 ����������� PH3 �ľ���Ч����ʱ��Ĺ�ϵ��ͼ��ʾ���ش��������⣺
H3PO4������������ͬʱ�� �ܽ�����Һ��O2 ����������� PH3 �ľ���Ч����ʱ��Ĺ�ϵ��ͼ��ʾ���ش��������⣺
����ͼ��֪������������______��ѡ��ӳ��������̡��������õij���ʱ�䡣
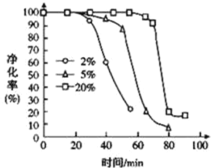
�����ŷ�Ӧ���У�PH3 �ľ���Ч�ʼ��罵�͵�ԭ�����Ϊ _________________ ��
���𰸡�![]() cd �� ���� PH4++OH-=PH3��+H2O 4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4 Ca3P2+6H2O=3Ca(OH)2+2PH3�� �ӳ� ���ɵ������ܺͽ���������Cu2+��Pd2+��Ӧ��ʹ��Ч�ʽ���
cd �� ���� PH4++OH-=PH3��+H2O 4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4 Ca3P2+6H2O=3Ca(OH)2+2PH3�� �ӳ� ���ɵ������ܺͽ���������Cu2+��Pd2+��Ӧ��ʹ��Ч�ʽ���
��������
��1��PH3������Pԭ�Ӻ�ÿ��Hԭ���γ�1�����ۼ�����Pԭ������㻹��һ���µ��Ӷԣ�
a������������IJ��غϵķ���Ϊ���Է��ӣ�
b�����ڵĻ�ѧ������Խ�����Խ�ȶ���
c��һ��PH3�����У�Pԭ���������һ�Թµ��Ӷԣ�
d������������⻯���۷е�ϸߣ�
��2����ΪNH3���Ӽ����������е㷴������Ϊ���ķǽ����Ա��ķǽ�����ǿ�����ĵ縺�Ա��ĵ縺��ǿ��������ˮ�γ���Һʱ��һˮ�ϰ��еĵ�ԭ�Ӹ���������ˮ�е������Ӷ��������������ʹ��Һ���Ը�ǿ���Ȼ��l��PH4C1����Ӧ����좵ķ�Ӧԭ�������Ȼ�����ķ�Ӧ��
��3������������ʾ��PH3 ��һ�ֻ�ԭ���� �ɽ�Cu2+����Һ��ԭ���ɽ���ͭ��������������Ϊ�������̬H3PO4���ݴ�д����ѧ����ʽ��
��4��Ca3P2�ڳ�ʪ�Ŀ����о��ҷ�Ӧ�����������ƺ�PH3��
��5���ٸ���ͼ֪�������ܽ����ԽС����Ӧ����Խ�죻
�����ɵ������ܺͽ��������ӷ�Ӧ��
��1��PH3������Pԭ��ͨ��3�����ۼ���3��Hԭ�����ϣ���ṹʽΪ![]() ��
��
a.PH3�����������ͷ��ӣ�����������IJ��غϵķ���Ϊ���Է��ӣ���a��ȷ��
b.Pԭ�ӵ�ԭ�Ӱ뾶����N���γ����������С�ڵ�������ܣ�����PH3 �����ȶ��Ե��� NH3���ӣ���b��ȷ��
c.һ�� PH3�����У�Pԭ�Ӻ����������һ�Թµ��Ӷԣ���c����
d.��ΪNH3���Ӽ�������������PH3 �е����NH3�е㣬������أ���d����
����![]() ��cd��
��cd��
��2����ΪNH3���Ӽ�������������PH3 �е���� NH3 �е㣻��Ϊ���ķǽ����Ա��ķǽ�����ǿ�����ĵ縺�Ա��ĵ縺��ǿ��������ˮ�γ���Һʱ��һˮ�ϰ��еĵ�ԭ�Ӹ���������ˮ�е������Ӷ��������������ʹ��Һ���Ը�ǿ������NH3 ��ˮ��Һ���Դ���PH3 ��ˮ��Һ���ԣ��Ȼ��l��PH4C1����Ӧ����좵ķ�Ӧԭ�������Ȼ�����ķ�Ӧ�������ӷ���ʽΪPH4++OH-=PH3��+H2O��
�����������ʴ�Ϊ���ͣ����ڣ�PH4++OH-=PH3��+H2O��
��3������������ʾ��PH3 ��һ�ֻ�ԭ���� �ɽ�Cu2+����Һ��ԭ���ɽ���ͭ�� ������������Ϊ�������̬H3PO4������PH3 �� CuSO4 ��Һ��Ӧ�Ļ�ѧ����ʽΪ4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4��
�ʴ�Ϊ��4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4��
��4��Ca3P2�����ڵ�ʯ��CaC2������ʯ��ˮ�ܷ�������ˮ��ķ�Ӧ�����������ƺ���Ȳ�������ڳ�ʪ�Ŀ�����Ca3P2��ˮ��Ӧ��Ӧ�ķ���ʽΪCa3P2+6H2O=3Ca(OH)2+2PH3����
�ʴ�Ϊ��Ca3P2+6H2O=3Ca(OH)2+2PH3����
��5������ͼ��֪���������������Խ��PH3�߾����ʳ���ʱ�䳤�����Ը����������ӳ������õij���ʱ�䣻
�ʴ�Ϊ���ӳ���
���������ŷ�Ӧ�Ľ��У���Ӧ����H3PO4�����Cu2+��Pd2+��Ӧ��ʹ��Ч�ʽ��ͣ�����PH3 �ľ���Ч�ʼ��罵�ͣ�
�ʴ�Ϊ�����ɵ������ܺͽ���������Cu2+��Pd2+��Ӧ��ʹ��Ч�ʽ��͡�