��Ŀ����
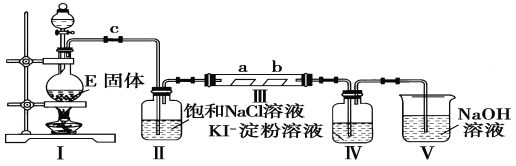
����Ŀ��I.ij��ѧ����С��������ͼһװ����ȡ�屽�������Һ©���� ���뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
��1���۲쵽A�е�������_________________________________��
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������ д���йط�Ӧ�Ļ�ѧ����ʽ_____________________________��
��3��C��ʢ��CCl4��������________________________________��
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ�����________________________________________��
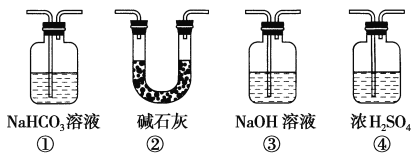
II.ʵ��������ͼ��ʾ��װ����ȡ����������

��1���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�������������£�__________��Ȼ������ʹ���Ͼ��ȡ�
��2��Ũ����������ǣ��� _______________���� ______________��
��3���ұ�װ����ͨ�����ĵ���Ҫ����Һ���϶����ܲ�����Һ�У�Ŀ���Ƿ�ֹ��Һ�ĵ�������ɵ�����ԭ����___________________��
��4������õ����������ķ�����________________��������Ҫ�IJ���������___________��
��5�����ӵ���C2H518OHд�������������ķ���ʽ________________________��
���𰸡� ��ӦҺ�У��к���ɫ�������A���� Br2+2NaOH=NaBr+NaBrO+H2O ��ȥ�廯�������е������� ʯ����Һ����Һ���ɫ ����Թ���ע�������Ҵ�����Ũ��������Ҵ��У��ӱ������������� ���� ��ˮ�� �ӷ������Ҵ�������������ˮ������ˮ����ѹǿ���������� ��Һ ��Һ©�����ձ� CH3COOH+CH3CH218OH====CH3CO18OCH2CH3+H2O
��������I.����Һ�������۴������·���ȡ����Ӧ�����屽���廯����C6H6+Br2![]() C6H5Br +HBr�����ڷ�Ӧ���ȣ�����Һ����ӷ������������屽���������廯��������ˮ�����H+��Br-�����������������ӻᷢ����Ӧ����AgBr������
C6H5Br +HBr�����ڷ�Ӧ���ȣ�����Һ����ӷ������������屽���������廯��������ˮ�����H+��Br-�����������������ӻᷢ����Ӧ����AgBr������
(1)���ڷ�Ӧ���ȣ�����Һ����ӷ�����������һ�ֺ���ɫ�����壬�ʴ�Ϊ����ӦҺ�У��к���ɫ�������A������
(2)A�е�����������Ʒ�Ӧ�����Խ��屽�е����ȥ����Br2+2NaOH=NaBr+NaBrO+H2O���ʴ�Ϊ��Br2+2NaOH=NaBr+NaBrO+H2O��
(3)������������ԭ�����弫���������Ȼ�̼�����廯�����ܣ�����C��ʢ��CCl4�������dz�ȥ�廯�������е����������ʴ�Ϊ����ȥ�廯�������е���������
(4)�������ȡ����Ӧ�������廯�⣬�廯��������ˮ�����H+��Br-��ֻҪ���麬�������ӻ������Ӽ��ɣ������ӵļ��飺ȡ��Һ�μ���������Һ��������ɵ���ɫ������֤���������ӣ������ӵļ��飺�����ʹ��ɫʯ����Һ��죬��֤�����������ӣ��ʴ�Ϊ��ʯ����Һ����Һ���ɫ��
II.(1)��ȡ�Ҵ��������Ũ����Ļ��Һʱ����ȷ��������Ϊ������Թ���ע�������Ҵ�����ŨH2SO4�����Ҵ��У��ӱ������������ᣬ�ʴ�Ϊ������Թ���ע�������Ҵ�����ŨH2SO4�����Ҵ��У��ӱ������������
(2)Ũ������������������ȡ��Ӧ�У��ܹ��ӿ췴Ӧ���ʣ����˴������ã��ܹ����շ�Ӧ���ɵ�ˮ��ͨ�������������IJ��ʣ�������ˮ�������ã��ʴ�Ϊ����������ˮ����
(3)�Ҵ�������ķе�ϵͣ���Ӧ���������ӷ������ڻӷ������Ҵ�������������ˮ������ˮ����ѹǿ��С���������ʴ�Ϊ���ӷ������Ҵ�������������ˮ������ˮ����ѹǿ��С��������
(4)�������������ڱ���̼������Һ�����Ի��Һ�ֲ㣬����ͨ����Һ�������������������������Һ����ʹ�õ������з�Һ©�����ձ��ȣ��ʴ�Ϊ����Һ����Һ©�����ձ���
(5)�������Ҵ��������������ķ�Ӧ�У��Ҵ���ȥ�ǻ��е���ԭ�ӡ�������ȥ�Ȼ��е��ǻ�������18O��Ӧ��������������У��÷�Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH218OH![]() CH3CO18OCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH218OH
CH3CO18OCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH218OH![]() CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O��

����Ŀ������������ĸߵͿɺ���һ�����һ�������ˮƽ��չ�ĸߵ���SO2ת��ΪSO3���Ʊ������еĹؼ��Է�Ӧ��Ҳ��һ�����淴Ӧ��
��1��NO����ΪSO2��O2�䷴Ӧ�Ĵ�����������������
��2NO(g)+O2(g)![]() 2NO2(g)��H1=-113kJ/mol
2NO2(g)��H1=-113kJ/mol
��SO2(g)+NO2(g)![]() SO3(g)+NO(g) ��H2
SO3(g)+NO(g) ��H2
�ܷ�Ӧ��2SO2(g)+O2(g)![]() 2SO3(g)��H3=-196.6kJ/mol ��H2=__________
2SO3(g)��H3=-196.6kJ/mol ��H2=__________
��2��һ���¶��������ݻ�Ϊ2L�ĺ����ܱ������г���2molSO2��2mo1O2���������ѹǿ�ı仯���±���ʾ(SO3Ϊ����)��
��Ӧʱ��/min | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
ѹǿ/MPa | 16.0a | 14.7a | 13.7a | 13.0a | 12.5a | 12.4a | 12.4a |
��0~10min������(SO2)=_________
�ڸ��¶��µ�ƽ�ⳣ��K=__(�������һλС��)��
�۷�Ӧ�ﵽƽ���������������ͬʱ�����Ϊ0.2mol���������������(��)����(��)�Ĺ�ϵ��___
��3��һ���¶�������ij�ܱ�������ͨ��һ�����Ķ�������������Ļ�����岢ʹ֮��Ӧ����Ӧ������SO2��O2��SO3���ʵ����仯����ͼ��ʾ��
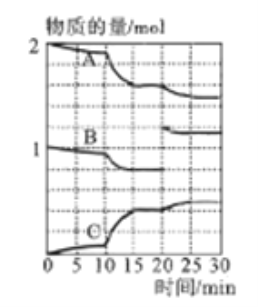
��A��B��C���������б�ʾSO2���ʵ����ı仯����__������15~20min��25~30min����ʱ����������ݻ�����������ijһʱ��SO3�ķֽ����ʽϴ��ʱ�����_______��
��10~15min�ڷ�Ӧ���ʷ��������Ա仯������Ե�ԭ����__________
(4)����������NaOH��Һ����SO2�ȿ�����SO2��ɵĴ�����Ⱦ��Ҳ�ɻ����Ҫ�Ļ�����Ʒ����ij����Һ��c(HSO3-):c(SO32-)=1:100����������Һ��pH=______(������K1(H2SO3)=1.5��10-2��K2(H2SO3)=1��10-7
����Ŀ��S2Cl2�����ĵ�������ճ����������ڵ�����ͨ������������Ӧ�Ƶã���һ���Ȼ��ɵ�SCl2��S2Cl2��SCl2��ijЩ�������£�
ˮ���� | �ܶ�(g/cm3) | ��ɫ | �۵� | �е� | |
S2Cl2 | �����з���������ˮ��ˮ�� | 1.687 | ���ɫ | -76�� | 138�� |
SCl2 | ����ˮ�Ҿ��ҷ�Ӧ | 1.621 | ӣ�Һ� | -122�� | 59�� |
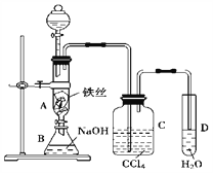
����ͼ��ʾװ���Ʊ�S2Cl2���ش��������⣺
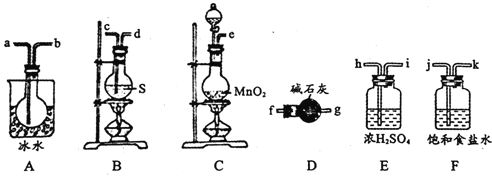
(1)��֪S2Cl2�����и�ԭ������������8�����ȶ��ṹ����S2Cl2�ĵ���ʽΪ________��
(2)��ȡCl2Ӧѡ�õ��b����_______(����ĸ���)����Ӧ�����ӷ���ʽΪ________��
(3)���õ��ϴ�����S2Cl2����������װ�õ�����˳��Ϊ____(������������Сд��ĸ��ʾ)��
(4)����D��������_______��D�м�ʯ�ҵ�������________��
(5)Ϊ�˻�ø�������S2Cl2����Ҫ�Բ�Ʒ���еIJ�����____________
(6)����S2Cl2����ˮ��ͬʱ�������������壬д����Ӧ�Ļ�ѧ����ʽ_________���÷�Ӧ�б������ͱ���ԭ��Ԫ�ص�����֮��Ϊ________________��