��Ŀ����
����Ŀ����������Ҫ��������Һ����ʾ����ƽ����Ļ����LCD����(��Ҫ�ɷ��Ǻ���������������)�л������Ĺ�����������:
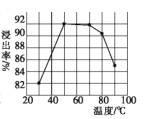
(1)����������������ʱ���������¶ȱ仯��ϵ��ͼ��ʾ,�������˵��¶���___________,�¶����߽������½���ԭ����__________
(2)���ʱ����������ת����In3+�����ӷ���ʽ��__________
(3)�ᴿ�����ķ�����ͭ�ľ���ԭ������,�����Ϊ__________(���������������),д�������ĵ缫��Ӧʽ: ______________________________
(4)�������������������ԭ��Ӧ����������__________(�ѧʽ),�������������������ԭ��Ӧ�����������뻹ԭ����ϵ��֮����__________
���𰸡�50���50~70�� �¶�����,����ӷ� In2O3+6H+==2In3++3H2O ���� In3++3e-===In H2O 1:1
��������
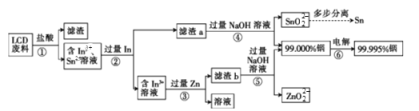
������ͼ��֪��������м����ᣬ���������������ܽ�����In3+��Sn2+�����������In������a�к�Ϊ�������Ļ���������м������������������Һ�����Խ�������ת��Ϊ������ˮ��SnO22-������b�к�Ϊ����п�Ļ���������м������������������Һ�����Խ�п����ת��Ϊ������ˮ��ZnO22-���ݴ˷�����
��1���������ʵĽ��������¶ȵĹ�ϵ��֪���¶���50����70��ʱ����������ߣ����ԡ�������������¶�Ϊ50����70�棻��Ϊ�¶����ߣ�����ӷ������������¶ȵ����ߣ������ʽ������ʴ�Ϊ��50���50��70�����¶�����������ӷ���
��2��������м����ᣬ���������������ܽ�����In3+��Sn2+����������������InΪ+3�ۣ���������Ļ�ѧʽΪIn2O3��������ת��ΪIn3+�����ӷ���ʽΪ��In2O3+6H+=2In3++3H2O���ʴ�Ϊ��In2O3+6H+=2In3++3H2O��
��3��������ʱ������Ϊ����������In3+������ԭ��Ӧ�ĵ缫����ʽΪIn3++3e-=In���ʴ�Ϊ��������In3++3e-=In��
��4��SnΪ���Խ���������������ƣ���������������Ʒ�Ӧ��2Al+2H2O+2NaOH=2NaAlO2+3H2��������Ҳ�������Ƶ�Na2SnO2�����Է�Ӧ����ʽΪ��Sn+2NaOH�TNa2SnO2+H2����Sn+2NaOH+2H2O�TNa2Sn(OH)4+H2����H2O����Ԫ�صĻ��ϼ��ɣ�1�۽���0�ۣ���������ΪH2O��ZnΪ���Խ���������������ƣ���Ӧ����ʽΪ��Zn+2NaOH�TNa2ZnO2+H2����Sn+2NaOH+2H2O�TNa2Zn(OH)4+H2������Ӧ��Na2ZnO2Ϊ��ԭ���H2Ϊ�������ϵ��֮����1:1���ʴ�Ϊ��H2O��1:1��

����Ŀ��������Ԫ��X��Y��Z��W�����ڱ��е����λ����ͼ��ʾ������WԪ�ص�ԭ�ӽṹʾ��ͼΪ![]() ��
��
W | X | Y |
Z |
��ش��������⣺
��1��ZԪ����Ԫ�����ڱ��е�λ����___________��
��2��X��Y��Z����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ___________��Ԫ�ط��ű�ʾ����
��3��X��Z��W����Ԫ�ص�����������Ӧˮ�����������ǿ����___________���û�ѧʽ��ʾ����
��4���õ���ʽ��ʾWY2���γɹ��̣�___________��
��5��д��W������Ũ���ᷴӦ�Ļ�ѧ����ʽ��___________��
����Ŀ����ȥ���������������������ʵķ�����ȷ���� (��������)
ѡ�� | ���� | ���� | �Լ� | �ᴿ���� |
A | ���� | ��ϩ | ���� | ���������� |
B | SiO2 | Al2O3 | NaOH��Һ | ���� |
C | �������� | ���� | NaOH��Һ | ��������÷�Һ |
D | BaSO4 | BaCO3 | ϡ���� | �ܽ⡢���ˡ�ϴ�� |
A.AB.BC.CD.D
