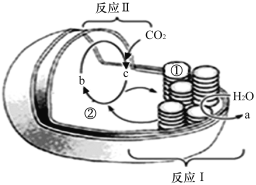
��Ŀ����
����Ŀ����һ����ҵ����CO2��H2��Ӧ�ϳɶ����ѡ���֪��
CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H1����49.1 kJ��mol��1
CH3OH(g)��H2O(g) ��H1����49.1 kJ��mol��1
2CH3OH(g)![]() CH3OCH3(g)��H2O(g) ��H2����24.5 kJ��mol��1
CH3OCH3(g)��H2O(g) ��H2����24.5 kJ��mol��1
��1��д��CO2(g)��H2(g)ת��ΪCH3OCH3(g)��H2O(g)���Ȼ�ѧ����ʽ_______________��
��2��������ȼ�ϵ�ؾ�������ת���ʸߡ���������ص�����㷺Ӧ�ã�һ�ֶ�����������أ������ΪKOH��Һ���ĸ�����ӦʽΪ��_______________________��
��3������2LNa2CO3��Һ��4.66 g BaSO4(233 g/moL)����ȫ��ת��ΪBaCO3�������õ�Na2CO3��Һ�����ʵ���Ũ������Ϊ__________________��[��֪��������Ksp(BaSO4)=1��10��11��Ksp (BaCO3)=1��10��10]����������Һ����ı仯��
��������20 mL���������Ļ����Һ�У���μ���0.05 mol��L��1Ba(OH)2��Һʱ�����ɳ����������仯���ɴ˶��������Һ��pH�ı仯��ͼ��ʾ��
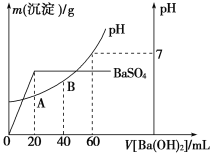
���㣺��1��ԭ�����Һ��c(Cl��)��________��
��2��A���pH��________��
���𰸡�2CO2(g)��6H2(g)![]() CH3OCH3(g)��3H2O(g) ��H����122.7 kJ��mol-1 CH3OCH3 ��12e- + 16OH- = 2CO32- + 11H2O 0.11mol/L 0.2 mol��L��1 1
CH3OCH3(g)��3H2O(g) ��H����122.7 kJ��mol-1 CH3OCH3 ��12e- + 16OH- = 2CO32- + 11H2O 0.11mol/L 0.2 mol��L��1 1
��������
��һ����1����˹���ɵ�Ӧ�ã�
��2��ȼ�ϵ���и���Ϊȼ��ʧ���ӣ�����Ϊ�����õ��ӣ�д���缫��Ӧʽ��
��3����Ͽ��淴Ӧ��ƽ�ⳣ�������ܵ���ʵ��ܽ�ƽ�ⳣ��һ���ǣ�
��������1������ͼ��֪���������ҺpH=7ʱ��˵�����������Ӻͼ������������ӵ����ʵ�����ȣ��ݴ˼���������Ũ�ȣ���20mLʱ�������������ǡ�÷�Ӧ�������ᱵ���ݴ˼������������Ũ�ȣ���ϵ���غ����������Ũ�ȣ�
��2������A����Һ��������Ũ�ȼ�����Һ��pH��
��1�����ø�˹���ɼ��㣬�� CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H1����49.1 kJ��mol��1���� 2CH3OH(g)
CH3OH(g)��H2O(g) ��H1����49.1 kJ��mol��1���� 2CH3OH(g)![]() CH3OCH3(g)��H2O(g) ��H2����24.5 kJ��mol��1��2����+�ڵó�2CO2(g)��6H2(g)
CH3OCH3(g)��H2O(g) ��H2����24.5 kJ��mol��1��2����+�ڵó�2CO2(g)��6H2(g)![]() CH3OCH3(g)��3H2O(g) ��H����122.7 kJ��mol-1��
CH3OCH3(g)��3H2O(g) ��H����122.7 kJ��mol-1��
��ȷ�𰸣�2CO2(g)��6H2(g)![]() CH3OCH3(g)��3H2O(g) ��H����122.7 kJ��mol-1��
CH3OCH3(g)��3H2O(g) ��H����122.7 kJ��mol-1��
��2��������������أ������ΪKOH��Һ������Ϊ���������������ѣ����Ի����µĸ�����ӦʽΪCH3OCH3 ��12e- + 16OH- = 2CO32- + 11H2O��
��ȷ�𰸣�CH3OCH3 ��12e- + 16OH- = 2CO32- + 11H2O��
��3��n(BaSO4)=4.66g/233 g/moL=0.02mol��BaSO4(233 g/moL)����ת��ΪBaCO3�Ļ�ѧ����ʽ��
BaSO4��s��+ CO32-��aq��![]() BaCO��s��+ SO42-��aq��
BaCO��s��+ SO42-��aq��
n������0.02 x 0 0
��n 0.02 0.02 0.02 0.02
n��ĩ��0 x-0.02 0.02 0.02
![]() = Ksp(BaSO4)./Ksp (BaCO3)= 1��10��11/(1��10��10)=1/10,�ɵ�x=0.22mol
= Ksp(BaSO4)./Ksp (BaCO3)= 1��10��11/(1��10��10)=1/10,�ɵ�x=0.22mol
C(Na2CO3)=0.22mol/2L=0.11mol/L��
��ȷ�𰸣�0.11��
��������1����ͼ��֪����pH=7ʱ������Ba��OH��2��Һ���Ϊ60mL������n��H+��=n��OH-������c��H+��=0.05mol/L��0.06L��2/0.02L=0.3molL-1��������20mLBa��OH��2��Һʱ�������������ȫ��Ӧ������ԭ���غ��c��SO42-��=c��H2SO4��=0.05mol/L��0.02L/0.02L=0.05mol/L��20 mL���������Ļ����Һ�и��ݵ���غ��c��Cl-��+2c��SO42-��=c��H+����c��Cl-��=c��H+��-2c��SO42-��=0.3molL-1-2��0.05mol/L=0.2molL-1��
��ȷ��:�� 0.2��
��2��A��c��H+��=(0.3mol/L��0.02L-2��0.05mol/L��0.02L)/0.04L=0.1mol/L������pH=1��
��ȷ�𰸣�1��

 �߽�������ϵ�д�
�߽�������ϵ�д�����Ŀ���״�CH3OH)��һ����Ҫ�Ļ���ԭ�ϣ���ҵ���ж��ַ������Ƶü״���Ʒ
(һ)��CO��H2��CO2�Ʊ��״�
��CO2(g)+H2(g)![]() COg)+H2O(g) H1
COg)+H2O(g) H1
��CO(g)+2H2 (g) ![]() CH3OH(g) ��H2
CH3OH(g) ��H2
��CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) H3
CH3OH(g)+H2O(g) H3
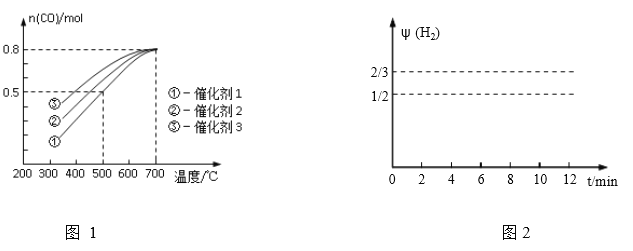
��1����֪:��Ӧ�ٵĻ�ѧƽ�ⳣ��K���¶ȵĹ�ϵ���±�
t/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
������˵����ȷ����______
A.��Ӧ������Ӧ�����ȷ�Ӧ
B.һ��������ܱ������У�ѹǿ���ٱ仯ʱ��˵����Ӧ�ٴﵽƽ��״̬
C.1100��ʱ����Ӧ�ٵ�K����Ϊ1.5
D.��1000��ʱ��[c(CO2)��c(H2)]/[c(CO)��c(H2O)]ԼΪ0.59
��2���Ƚ���H2_____��H3(����>������������������)
��3�������âں͢�������Ӧ�ϳ�CH3OH����֪CO��ʹ��Ӧ�Ĵ��������½�����̼�ȱ�ʾΪf��[n(H2)-n(CO2)]/[n(CO)+n(CO2)]����������f��_____ʱ��ԭ�����������ʸߣ���������ס�������Ը��ڸ�ֵ����̼�ȣ�������_________________________________.
(��)����Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״�:
(i)�Ʊ��ϳ���:CH4(g)+H2Og) ![]() CO(g)+3H2(g) H1>0
CO(g)+3H2(g) H1>0
(ii)�ϳɼ״�:CO(g)+2H2(g) ![]() CH3OH(g) H2>0
CH3OH(g) H2>0
��һ��ѹǿ�£�1 mol CH4(g)��1 mol H2O(g)�����ֲ�ͬ���������·�����Ӧ(i)��������ͬʱ��ʱ��CO�����ʵ���(n)���¶ȱ仯�Ĺ�ϵ��ͼ1
��1������˵����ȷ����_______
A.���ߢ���n(CO)���¶ȱ仯��ԭ��������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�
B.���ִ����У������۵Ĵ�Ч����ã������ܻ����ߵIJ���
C.���¶ȵ���700��ʱ�������ϵĵ���ܶ�û�е���ƽ��
D.���¶ȴ���700��ʱ��CO�����ʵ������ֲ���
��2��500��ʱ����Ӧ(1)�ڴ����ٵ������µ�10mimʱ�ﵽƽ�⣬����ͼ2�л�����Ӧ��1���ڴ�״̬��0��12�����ڷ�Ӧ��ϵ��H2���������![]() (H2)��ʱ��t�仯��������___________________
(H2)��ʱ��t�仯��������___________________
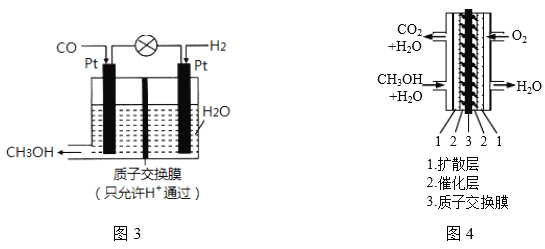
�������о�������COҲ��������������ͨ���绯ѧ�ķ����Ʊ��״���ԭ����ͼ3��ʾ��
��1�������״��ĵ缫��ӦʽΪ___________________��
��2���״�ȼ�ϵ��Ӧ�úܹ㣬�乤��ԭ����ͼ4��д����ع���ʱ�ĸ�����Ӧʽ:___________��
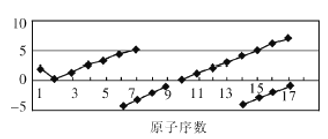
����Ŀ����ͼ�������������Ƿ��ֿ�ѧ���ɵ�һ����Ҫ������
��1����ͼ��ԭ������Ϊ1��18��Ԫ��ԭ�ӵ�������������ԭ�������仯��ֱ��ͼ��ͼ��Ԫ��a ��__��b ��____��

��2����ͼ��ʾԪ�ص�һ��������ԭ�������仯����������ݷ�������ͼ�������ʾ����_________��
��3���±������˲���Ԫ�ص�ԭ�Ӱ뾶��
Ԫ�ط��� | Li | Be | B | C | N | O | F | Na | K | Rb | Cs |
ԭ�Ӱ뾶/nm | 0.152 | 0.089 | 0.082 | 0.077 | 0.075 | 0.074 | 0.071 | 0.186 | 0.227 | 0.248 | 0.265 |
����ݱ������ݷ���ͬ����Ԫ��ԭ�Ӱ뾶�ĵݱ������______��ͬ����Ԫ��ԭ�Ӱ뾶�ĵݱ������_____���ݱ��еó��Ĺ��ɱȽ�Ca2+��Cl-�İ뾶��С��r(Ca2+)_____r(Cl-)������>����<��������������