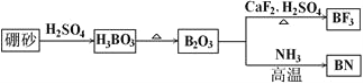
��Ŀ����
����Ŀ����20����60����Ժ����Ƿ�����120���ֺ������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��ø�͵����ʡ����Ǵ����������������ϵ���������֮һ��ij��ѧ��ȤС�����о�ij����ؽṹ�����ʱ�����������ʵ�飺
��ø�͵����ʡ����Ǵ����������������ϵ���������֮һ��ij��ѧ��ȤС�����о�ij����ؽṹ�����ʱ�����������ʵ�飺
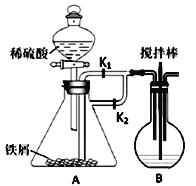
ʵ��һ���ⶨ���������
(1)����װ�ã�����д�ӿ�˳��b��____________________
(2)���װ�õ������ԣ���A�з���0.4g����ص���Ʒ![]() ���в�����ˮ�����������
���в�����ˮ�����������![]() ,��B�м���Ʒ����Һ����C�м���30mL 0.1mol/L������
,��B�м���Ʒ����Һ����C�м���30mL 0.1mol/L������![]() ��Һ��
��Һ��
(3)ͨ����������ȣ����ֹ�����ת��Ϊ����ɫ��
(4)��������ȫת����ȡC�е�![]() ��Һ
��Һ![]() ����0.1mol/L�ĵ⻯��
����0.1mol/L�ĵ⻯��![]() ��Һ���еζ�����¼�������£�
��Һ���еζ�����¼�������£�
�ζ����� | ������Һ��� | ���ĵ⻯����Һ��� | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
ʵ������ⶨ����������
ȡʵ������A��Ӳ�ʲ������еIJ����������ϡ�����У���ֽ������ˣ�����Һ�м���������NaOH��Һ�����˺�ȡ�����������յ�0.32g���壮�Իش��������⣺
(1)�����ʵ��һ����װ��A�������Եķ�����_________
(2)�ζ��յ���жϷ�����_________
(3)װ��B��Ʒ����Һ��������_______![]() ��ͬѧ�������ȥBװ�ã���ʵ��û��Ӱ�죬��Ŀ�����______
��ͬѧ�������ȥBװ�ã���ʵ��û��Ӱ�죬��Ŀ�����______![]() ѡ����������������������
ѡ����������������������![]() ��������_________
��������_________
(4)��KI��Һ�ζ�![]() ��Һʱ������Ӧ�����ӷ���ʽΪ_________
��Һʱ������Ӧ�����ӷ���ʽΪ_________
(5)�������������ؽṹ�Ļ�ѧʽ_________
(6)���в�������������![]() ƫ�����_________
ƫ�����_________
![]() �ζ�ʣ��
�ζ�ʣ��![]() ��Һʱ��KI��Һ�ε���ƿ���һ��
��Һʱ��KI��Һ�ε���ƿ���һ��
![]() ����KI��Һʱ������ʱ���ӿ̶���
����KI��Һʱ������ʱ���ӿ̶���
![]() �õ⻯����Һ�ζ�ʣ��
�õ⻯����Һ�ζ�ʣ��![]() ��Һʱ���ζ�ǰ�����ݣ��ζ���������
��Һʱ���ζ�ǰ�����ݣ��ζ���������
![]() ʵ����У����������ղ����
ʵ����У����������ղ����
���𰸡�b��![]() д��д����
д��д����![]() �ڵ���b���ϳ����ܣ���ĩ�˲���ˮ���У��رջ������þƾ�����Ӳ���Թ�A�����ܳ����ܿ������ݲ�������ȥ�ƾ��ƣ������γ�һ��ˮ����˵��װ������������ �������һ��KI��Һ����Һ��ɫ��ȥ���Ұ���Ӳ��ָ�Ϊ��ɫ ������������Ƿ����Ը��������ȫ���� ���� ��B�и��������Һ����ɫ����ȥ��˵����������������ȫ
�ڵ���b���ϳ����ܣ���ĩ�˲���ˮ���У��رջ������þƾ�����Ӳ���Թ�A�����ܳ����ܿ������ݲ�������ȥ�ƾ��ƣ������γ�һ��ˮ����˵��װ������������ �������һ��KI��Һ����Һ��ɫ��ȥ���Ұ���Ӳ��ָ�Ϊ��ɫ ������������Ƿ����Ը��������ȫ���� ���� ��B�и��������Һ����ɫ����ȥ��˵����������������ȫ ![]()
![]()
![]()
��������
����ص���Ʒ��װ������O2��Ӧ���õ�SO2�����SO2�ĺ����������Ը���������գ�����Ʒ����Һ���SO2�Ƿ�������ȫ���ٽ�β�����ա�
ʵ��һ��(1)�ø���������ն���������Ʒ��֤����������������ȫ�������������������β�����ӿ�˳��b��![]() д��д����
д��д����![]() ���ʴ�Ϊ��b��
���ʴ�Ϊ��b��![]() д��д����
����![]() ��
��
ʵ�����(1)���γ�һ�ܱ���ϵ�����ü������ͷ����װ�������ԣ��ʴ�Ϊ���ڵ���b���ϳ����ܣ���ĩ�˲���ˮ���У��رջ������þƾ�����Ӳ���Թ�A�����ܳ����ܿ������ݲ�������ȥ�ƾ��ƣ������γ�һ��ˮ����˵��װ�����������ã�
(2)�⻯��ʹ���������Һ��ɫ���ʴ�Ϊ���������һ��KI��Һ����Һ��ɫ��ȥ���Ұ���Ӳ��ָ�Ϊ��ɫ��
(3)Ʒ���Ǽ�����������Ƿ����Ը��������ȫ���գ�Ҳ��ʡȥƷ��װ�ã�����Ը��ݸ��������ɫ�仯��ȷ���Ƿ���ȫ���գ���B�и��������Һ����ɫ����ȥ��˵����������������ȫ���ʴ�Ϊ��������������Ƿ����Ը��������ȫ���գ���������B�и��������Һ����ɫ����ȥ��˵����������������ȫ��
(4)�������������ԭΪ�����ӣ������ӱ�����Ϊ�ⵥ�ʣ��ʴ�Ϊ��![]() ��
��
(5)��һ�εζ���������Һ��������2��3�����̫��Ӧ��ȥ��ȡ��2��3�ε�ƽ��ֵ�������Һ�����Ϊ![]() �����ݻ�ѧ����ʽ��
�����ݻ�ѧ����ʽ��![]() ֪ʣ��ĸ��������
֪ʣ��ĸ��������![]() ��������30mL��ֻȡ��3mL�����Թ�ʣ��������
��������30mL��ֻȡ��3mL�����Թ�ʣ��������![]() �����Բμӷ�Ӧ�ĸ��������
�����Բμӷ�Ӧ�ĸ��������![]() ���ٸ��ݹ�ϵʽ
���ٸ��ݹ�ϵʽ![]() �������ɶ�������
�������ɶ�������![]() ��ȡʵ������A��Ӳ�ʲ������еIJ����������ϡ�����У���ֽ������ˣ�����Һ�м���������NaOH��Һ�����˺�ȡ�����������յ�
��ȡʵ������A��Ӳ�ʲ������еIJ����������ϡ�����У���ֽ������ˣ�����Һ�м���������NaOH��Һ�����˺�ȡ�����������յ�![]() ���壬������
���壬������![]() ������
������![]() ��������Ԫ�غ���Ԫ���غ�֪
��������Ԫ�غ���Ԫ���غ�֪![]() ��
��![]() ��5��ȷ��
��5��ȷ��![]() �Ļ�ѧʽΪ��
�Ļ�ѧʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)a.�ζ�ʣ��![]() ��Һʱ��KI��Һ�ε���ƿ���һ�����Һƫ�࣬����ʱ������Ԫ��ƫ�٣���ֵƫ��
��Һʱ��KI��Һ�ε���ƿ���һ�����Һƫ�࣬����ʱ������Ԫ��ƫ�٣���ֵƫ��
b.����KI��Һʱ������ʱ���ӿ̶��ߣ���ʹŨ��ƫ����ʣ��������ƫС����ƫ�࣬��ֵƫС��
c.�õ⻯����Һ�ζ�ʣ��![]() ��Һʱ���ζ�ǰ�����ݣ��ζ��������ݵ��±�Һ������ƫ��ʣ��ĸ������ƫ�࣬�������Ԫ��ƫ�٣���ֵƫ��
��Һʱ���ζ�ǰ�����ݣ��ζ��������ݵ��±�Һ������ƫ��ʣ��ĸ������ƫ�࣬�������Ԫ��ƫ�٣���ֵƫ��
d.ʵ����У����������ղ���ֻ�������Ԫ��ƫ�࣬��ֵƫ��
acd��ȷ���ʴ�Ϊ��acd��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
��. [��������]
��1��Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
��2����Na2CO3��Na2S���Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ�г���������Na2SO3��Na2SO4��
��3��Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��4�������������ⷴӦ�����ӷ���ʽΪ��2![]() +I2=
+I2=![]() +2I
+2I
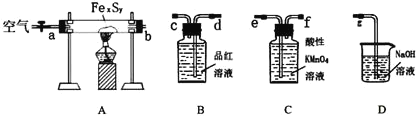
��. [�Ʊ���Ʒ]ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)
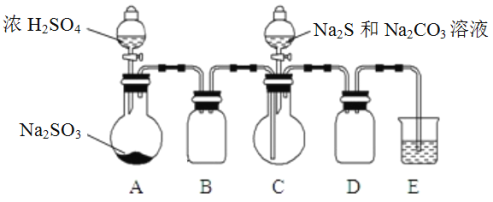
ʵ�鲽�裺
��1������ͼ��ʾ��װ��װ�ú�Ӧ��_________________________________________(���������)���ٰ�ͼʾ�����Լ�������B��D��������__________________________________________��
��2��������ƿC�м���Na2S��Na2CO3�����Һ��������ƿA�еμ�ŨH2SO4��C�з�Ӧ����Na2S2O3��CO2����ѧ����ʽΪ____________________________________________________________________��
��3����Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л��Һ����Һ���������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��. [̽���뷴˼]
��4����I2�ı���Һ�ⶨ��Ʒ�Ĵ��ȡ�ȡ10.0g��Ʒ�����Ƴ�100mL��Һ��������Һ������ˮ���뾭����С���ȴ�����ʹ�ã���Ŀ����ɱ������_______________________________��������̼��ȡ10.00mL��Һ����________________________________��ҺΪָʾ������Ũ��Ϊ0.10 mol/L I2�ı���Һ���еζ���������ݼ�¼���±���ʾ��
��� | 1 | 2 | 3 |
��Һ�����/mL | 10.00 | 10.00 | 10.00 |
����I2����Һ�����/mL | 19.95 | 17.10 | 20.05 |
�ζ�ʱ���ﵽ�ζ��յ��������___________________________��Na2S2O3��5H2O�ڲ�Ʒ�е�����������____________________________________(�ðٷ�����ʾ���ұ���1λС��)��