��Ŀ����
����Ŀ������ͼװ���Ʊ�FeCO3����ȡ����FeCO3���������ᷴӦ�ɵ�����������������֪��������������(C6H11O7)2Fe�dz��õIJ�������������ˮ���ش��������⣺
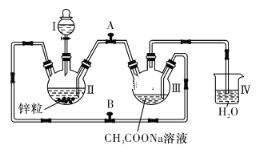
��(1)B�м���ҩƷNa2CO3��ҪʹA���Ƶõ�FeSO4��Һ����B�з�����Ӧ��ʵ�鿪ʼʱ��Ӧ��Һ©���Ļ�����______���ر�______��һ��ʱ��ر�_______����________ (�������ı��)��
(2)������������̼��������ϣ���Ӧ�Ļ�ѧ����ʽΪ(�л����÷���ʽ��ʾ)________________��
(3)�ӻ��������ĽǶȷ�������װ�ô��ڲ���֮���ǣ�_____________��
��.ij����������Ҫ�ɷ���������������������ԭ�ζ����ɲⶨ�ò���������Ԫ�صĺ�����ʵ�����Ҫ�������£�
��ȡ10Ƭ��������Ʒ���ܽ��ȥ������(�������Ԫ��)�������100mL������Һ��
����ȡ25.00mL����Һ����ƿ�С�
����c mol��L1������KMnO4��Һ�ζ����յ㣬��¼����KMnO4��Һ��������ظ�����ʵ�飬ƽ������KMnO4��Һ���ΪV mL��
(4)�õζ�ԭ�������ӷ���ʽΪ______________________
(5)����ʵ����Ӧ����ϡ�����ữKMnO4��Һ������������KMnO4��Һ�����ữ���Բⶨ�����Ӱ����________(����ƫ������ƫС��������Ӱ����)���ζ��յ��ʵ������Ϊ______��
(6)ÿƬ����������Ԫ�ص�����Ϊ__________g(�ô���ʽ��ʾ)��
���𰸡�K2 K1 K2 K1 2C6H12O7+ FeCO3�� (C6H11O7)2Fe + CO2 �� + H2O ��β������װ�� MnO4�� + 5Fe2+ +8H+ = 5Fe3+ + Mn2++4H2O ƫС ���������һ�θ��������Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���Ϊԭ����ɫ 0.112cV
��������
(1)����Aװ���Ʊ����������������������ų�װ���ڵĿ������ٽ�A����������ѹ�뵽B�еõ�̼��������
(2)������������̼��������������������������Ͷ�����̼��ˮ��
(3)�ӻ��������ĽǶȷ�����û��β������װ�á�
(4)����������ԭ��Ӧ�õ��õζ�ԭ�������ӷ���ʽ��
(5)����������������ӣ���������������١�
(6)���ݷ�Ӧԭ����ʽ���м��㡣
(1)����Aװ���Ʊ����������������������ų�װ���ڵĿ���������������������ѹǿ��A����������ѹ�뵽B�еõ�̼��������ʵ�鿪ʼʱ��Ӧ��Һ©���Ļ�����K2���ر�K1��һ��ʱ��ر�K2����K1���ʴ�Ϊ��K2��K1��K2��K1��
(2)������������̼��������ϣ���Ӧ�Ļ�ѧ����ʽΪ 2C6H12O7+ FeCO3�� (C6H11O7)2Fe + CO2 �� + H2O���ʴ�Ϊ��2C6H12O7+ FeCO3�� (C6H11O7)2Fe + CO2 �� + H2O��
(3)�ӻ��������ĽǶȷ�������װ�ô��ڲ���֮���ǣ���β������װ�ã��ʴ�Ϊ����β������װ�á�
(4)Fe2+���л�ԭ�ԣ�MnO4-����ǿ�����ԣ��õζ�ԭ�������ӷ���ʽΪMnO4�� + 5Fe2+ +8H+ = 5Fe3+ + Mn2++4H2O���ʴ�Ϊ��MnO4�� + 5Fe2+ +8H+ = 5Fe3+ + Mn2++4H2O��
(5)����ʵ����Ӧ����ϡ�����ữKMnO4��Һ������������KMnO4��Һ�����ữ������������������ӣ���������������٣��Բⶨ�����Ӱ����ƫС���ζ��յ��ʵ������Ϊ���������һ�θ��������Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���Ϊԭ����ɫ���ʴ�Ϊ��ƫС�����������һ�θ��������Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���Ϊԭ����ɫ��
(6)
MnO4�� ~ 5Fe2+
1mol 5mol
c mol��L1��V��10-3L n��Fe2+��
1mol����c mol��L1��V��10-3L ��= 5mol��n��Fe2+��
n��Fe2+��=5cV��10-3mol
ÿƬ����������Ϊ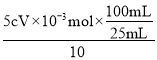 ��56g/mol=0.112cVg���ʴ�Ϊ��0.112cV��
��56g/mol=0.112cVg���ʴ�Ϊ��0.112cV��

����Ŀ����20����60����Ժ����Ƿ�����120���ֺ������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��ø�͵����ʡ����Ǵ����������������ϵ���������֮һ��ij��ѧ��ȤС�����о�ij����ؽṹ�����ʱ�����������ʵ�飺
��ø�͵����ʡ����Ǵ����������������ϵ���������֮һ��ij��ѧ��ȤС�����о�ij����ؽṹ�����ʱ�����������ʵ�飺
ʵ��һ���ⶨ���������
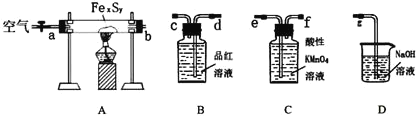
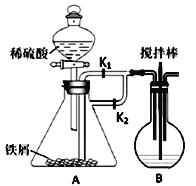
(1)����װ�ã�����д�ӿ�˳��b��____________________
(2)���װ�õ������ԣ���A�з���0.4g����ص���Ʒ![]() ���в�����ˮ�����������
���в�����ˮ�����������![]() ,��B�м���Ʒ����Һ����C�м���30mL 0.1mol/L������
,��B�м���Ʒ����Һ����C�м���30mL 0.1mol/L������![]() ��Һ��
��Һ��
(3)ͨ����������ȣ����ֹ�����ת��Ϊ����ɫ��
(4)��������ȫת����ȡC�е�![]() ��Һ
��Һ![]() ����0.1mol/L�ĵ⻯��
����0.1mol/L�ĵ⻯��![]() ��Һ���еζ�����¼�������£�
��Һ���еζ�����¼�������£�
�ζ����� | ������Һ��� | ���ĵ⻯����Һ��� | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
ʵ������ⶨ����������
ȡʵ������A��Ӳ�ʲ������еIJ����������ϡ�����У���ֽ������ˣ�����Һ�м���������NaOH��Һ�����˺�ȡ�����������յ�0.32g���壮�Իش��������⣺
(1)�����ʵ��һ����װ��A�������Եķ�����_________
(2)�ζ��յ���жϷ�����_________
(3)װ��B��Ʒ����Һ��������_______![]() ��ͬѧ�������ȥBװ�ã���ʵ��û��Ӱ�죬��Ŀ�����______
��ͬѧ�������ȥBװ�ã���ʵ��û��Ӱ�죬��Ŀ�����______![]() ѡ����������������������
ѡ����������������������![]() ��������_________
��������_________
(4)��KI��Һ�ζ�![]() ��Һʱ������Ӧ�����ӷ���ʽΪ_________
��Һʱ������Ӧ�����ӷ���ʽΪ_________
(5)�������������ؽṹ�Ļ�ѧʽ_________
(6)���в�������������![]() ƫ�����_________
ƫ�����_________
![]() �ζ�ʣ��
�ζ�ʣ��![]() ��Һʱ��KI��Һ�ε���ƿ���һ��
��Һʱ��KI��Һ�ε���ƿ���һ��
![]() ����KI��Һʱ������ʱ���ӿ̶���
����KI��Һʱ������ʱ���ӿ̶���
![]() �õ⻯����Һ�ζ�ʣ��
�õ⻯����Һ�ζ�ʣ��![]() ��Һʱ���ζ�ǰ�����ݣ��ζ���������
��Һʱ���ζ�ǰ�����ݣ��ζ���������
![]() ʵ����У����������ղ����
ʵ����У����������ղ����