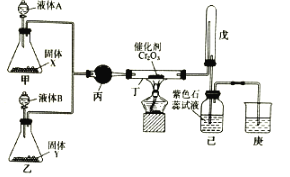
��Ŀ����
����Ŀ��I.�̵����е�NO2����Ҫ�Ĵ�����Ⱦ��֮һ��Ϊ�˼���京����ѡ�����¼�ⷽ�����ش��������⣺
��VL����ͨ�������ữ��H2O2��Һ�У�ʹNO2��ȫ��������NO3-����ˮϡ����100.00mL����ȡ20.00mL����Һ������v1mLc1mol��L��1 FeSO4����Һ(����)����ַ�Ӧ����c2mol��L��1 K2Cr2O7����Һ�ζ�ʣ���Fe2�����յ�ʱ����v2mL��
(1)NO2��H2O2����ΪNO3-�����ӷ���ʽΪ____________________________________��
(2)��ˮϡ�͵�100.00ml���õIJ�����������Ͳ���ձ�������������ͷ�ι��⣬����Ҫ__________________��
(3)�ζ������з������з�Ӧ��
3Fe2����NO3-��4H��=NO����3Fe3����2H2O
Cr2O72-��6Fe2����14H��=2Cr3����6Fe3����7H2O
��������NO2�ĺ���Ϊ___________mg/L��
(4)���в�����ʹ�ζ����ƫ�ߵ���____
A���ζ���δ�ñ�Һ��ϴ B����ƿϴ������������ˮ
C���ζ��ܵζ�ǰ������ȷ���ζ����Ӷ��� D��FeSO4����Һ���ֱ���
II�������£��÷�̪��ָʾ������0.10mol��L��1 NaOH��Һ�ֱ�ζ�20.00mLŨ�Ⱦ�Ϊ0.10mol��L��1�� CH3COOH��Һ��HCN��Һ���õζ�������ͼ��
����֪��CH3COOH�� HCN�ĵ���ƽ�ⳣ���ֱ�Ϊ1.75��10-5��6.4��10-10��
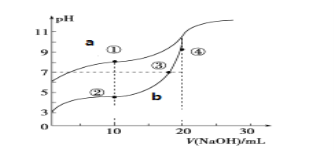
(1)ͼ___��a��b����NaOH��Һ�ζ�HCN��Һ��pH�仯�����ߣ��жϵ�������_______________________��
(2)������ʾ��Һ����������Ũ�ȵĴӴ�С��˳��_______________________��
(3)�����͵�����ʾ��Һ�У�c(CH3COO��)��c(CN��)___c(HCN)��c(CH3COOH)������>��<��=����
(4)���ڢۢ���ʾ����Һ��ˮ�ĵ���̶��ɴ�С��˳���ǣ�____________________��
���𰸡�2NO2��H2O2= 2NO3����2H�� 100mL����ƿ ![]() CD a HCN�ĵ���ƽ�ⳣ��С��ͬŨ�ȣ���������������Ũ��С��pHֵ�� c(CH3COO��)= c(Na+) > c(OH��) = c(H+) = �ܢۢ�
CD a HCN�ĵ���ƽ�ⳣ��С��ͬŨ�ȣ���������������Ũ��С��pHֵ�� c(CH3COO��)= c(Na+) > c(OH��) = c(H+) = �ܢۢ�
��������
I(1)NO2��H2O2����ΪNO3�������ӷ���ʽΪ2NO2��H2O2= 2NO3����2H����
(2)��ˮϡ�͵�100.00ml���õIJ�����������Ͳ���ձ�������������ͷ�ι��⣬����Ҫ100mL����ƿ��
(3)�ȼ����ظ�����������ĵ��������ӵ����ʵ������ټ�����������ĵ��������ӵ����ʵ������ټ�����������ʵ������������������ʵ������ټ���vL�����ж��������ĺ�����
(4)Aѡ��ζ���δ�ñ�Һ��ϴ�����ĵñ�Һ��������ظ�����������ƫ���ظ����������������ƫ�������������������ƫС���ζ����ƫ�ͣ�Bѡ���ƿϴ������������ˮ���ⶨ������䣻Cѡ��ζ��ܵζ�ǰ������ȷ���ζ����Ӷ���ƫС���ظ����������������ƫС�������������������ƫ�ζ����ƫ�ߣ�Dѡ�FeSO4����Һ���ֱ��ʣ��ⶨ�ظ�������ƫС���ظ����������������ƫС�������������������ƫ�ⶨ���ƫ�ߡ�
II��(1)ͼ���ݵ���ƽ�ⳣ����֪��ͬŨ��HCN��pH����̶�С��pH���������a��NaOH��Һ�ζ�HCN��Һ��pH�仯�����ߡ�
(2)�����Ǵ������������Ʒ�Ӧ�����ԣ����ݵ���غ����Һ�����Եó�����Ũ�ȵĴӴ�С��˳��
(3)��������ΪNaCN��HCN��Ũ����ȣ���������ΪCH3COOH��CH3COONa��Ũ����ȣ����������������ʵ�Ũ�ȶ���ͬ�����������غ�ñ��εó����ۡ�
(4)�ӵ��������������У���Һ�������ٷ�������кͷ�Ӧ�����ˮ�ĵ���̶���С����ˮ�ĵ���̶�������
I(1)NO2��H2O2����ΪNO3�������ӷ���ʽΪ2NO2��H2O2= 2NO3����2H�����ʴ�Ϊ��2NO2��H2O2= 2NO3����2H����
(2) ��ˮϡ�͵�100.00ml���õIJ�����������Ͳ���ձ�������������ͷ�ι��⣬����Ҫ100mL����ƿ���ʴ�Ϊ��100mL����ƿ��
(3)Cr2O72���� 6Fe2����14H��=2Cr3����6Fe3����7H2O
1mol 6mol
c2mol��L��1��v2��10-3 L xmol
![]()
x = 6c2v2��10-3 mol
�������������ʵ���n(FeSO4) = c1mol��L��1��v1��10-3 L= c1v1��10-3 mol
����������������������ʵ���Ϊn(FeSO4) = c1v1��10-3 mol - 6c2v2��10-3 mol =(c1v1- 6c2v2) ��10-3 mol
3Fe2���� NO3����4H��=NO����3Fe3����2H2O
3mol 1mol
(c1v1- 6c2v2) ��10-3 mol ymol
![]()
![]()
���VL������NO2�����ʵ���![]() ��������NO2������Ϊ
��������NO2������Ϊ![]() =
=![]() ����������NO2���
����������NO2�ĺ���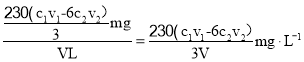 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(4)Aѡ��ζ���δ�ñ�Һ��ϴ�����ĵñ�Һ��������ظ�����������ƫ���ظ����������������ƫ�������������������ƫС���ζ����ƫ�ͣ�
Bѡ���ƿϴ������������ˮ���ⶨ������䣻
Cѡ��ζ��ܵζ�ǰ������ȷ���ζ����Ӷ���ƫС���ظ����������������ƫС�������������������ƫ�ζ����ƫ�ߣ�
Dѡ�FeSO4����Һ���ֱ��ʣ��ⶨ�ظ�������ƫС���ظ����������������ƫС�������������������ƫ�ⶨ���ƫ�ߣ�
�ʴ�ΪCD��
II��(1)ͼ���ݵ���ƽ�ⳣ����֪��ͬŨ���£�HCN�ĵ���̶�С��pH���������a��NaOH��Һ�ζ�HCN��Һ��pH�仯�����ߣ��ʴ�Ϊ��a��HCN�ĵ���ƽ�ⳣ��С��ͬŨ�ȣ���������������Ũ��С��pHֵ��
(2)�����Ǵ������������Ʒ�Ӧ�����ԣ����ݵ���غ����Һ�����Եó�����Ũ�ȵĴӴ�С��˳��c(CH3COO��)= c(Na+) > c(OH��) = c(H+)���ʴ�Ϊ��c(CH3COO��)= c(Na+) > c(OH��) = c(H+)��
(3)��������ΪNaCN��HCN��Ũ����ȣ���������ΪCH3COOH��CH3COONa��Ũ����ȣ����������������ʵ�Ũ�ȶ���ͬ�����������غ�ñ��εó���c(CH3COO��)��c(CN��) = c(HCN)��c(CH3COOH)���ʴ�Ϊ��=��
(4)�ӵ��������������У���Һ�������ٷ�������кͷ�Ӧ�����ˮ�ĵ���̶���С����ˮ�ĵ���̶������������Һ��ˮ�ĵ���̶��ɴ�С��˳���ǣ��ܢۢ����ʴ�Ϊ���ܢۢ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�