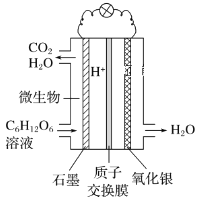
题目内容
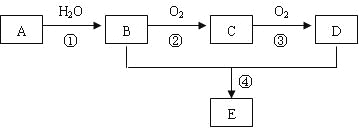
【题目】实验小组利用Na2SO3和浓硫酸制备SO2并进行相关实验探究。实验方案如图。
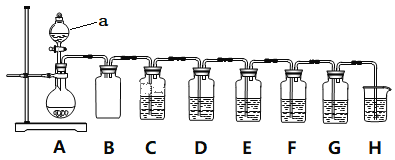
已知:各装置中的试剂,C(品红溶液)、D(酸性KMnO4溶液)、E(H2S溶液)、F(淀粉I2水溶液)、G(H2O2和BaCl2混合液)
(1)仪器组装完后首先要进行的操作是____________________。
(2)仪器a的名称是________________;装置B的作用_____________________。
(3)装置A中发生反应的化学方程式____________________。
(4)装置C和G中的现象:C___________________;G___________________;
(5)设计装置D和E的目的是验证SO2的___________性和___________性。
(6)装置H中的试剂是______________;其作用是_____________________。
(7)F中反应的离子方程式____________________。
【答案】检验装置的气密性 分液漏斗 防止倒吸,缓冲气流 Na2SO3+H2SO4=Na2SO4+H2O+SO2↑ 褪色 产生白色沉淀(或变浑浊) 还原 氧化 NaOH溶液 尾气处理 SO2+I2+2H2O=SO42-+2I-+4H+
【解析】
有气体参加的反应或制取气体的装置在仪器组装完后首先要检查装置的气密性,然后再加入药品进行实验。在A中制取SO2,B是安全瓶,C检验SO2的存在,D验证SO2的还原剂,E验证SO2的氧化性,F验证SO2的还原性,在G是H2O2将SO2氧化为硫酸,硫酸与BaCl2发生复分解反应产生BaSO4沉淀,装置H用NaOH溶液金属尾气处理,防止污染大气,据此分析解答。
(1)该装置要制取SO2,并验证其性质,因此仪器组装完后首先要进行的操作是检查装置气密性是否良好,只有装置不漏气,才可以装入药品进行实验。
(2)根据仪器结构,可知仪器a的名称是分液漏斗;装置B的作用安全瓶,防止倒吸现象的发生,同时可以使气体平稳,产生稳定的SO2气流,起缓冲气流的作用;
(3)在装置A中Na2SO3与硫酸发生复分解反应产生SO2气体,反应的化学方程式为:Na2SO3+H2SO4=Na2SO4+H2O+SO2↑。
(4)SO2具有漂白性,可以使装置C中的品红溶液褪色;G中的H2O2具有强的氧化性,能将SO2氧化为硫酸,H2SO4与BaCl2溶液反应产生BaSO4白色沉淀和HCl,因此看到的现象是:C中品红溶液褪色;G中溶液变浑浊;
(5)装置D中KMnO4溶液具有强氧化性,会将SO2氧化为硫酸,证明SO2具有还原性;装置E中含有H2S,H2S与SO2反应产生难溶于水的S和H2O,看到E中变浑浊,证明了SO2的氧化性。
(6)SO2是大气污染物,不能直接排放,否则会污染环境,所以装置H的作用是尾气处理,其中的试剂是NaOH溶液,反应的方程式为SO2+2NaOH=Na2SO3+H2O。
(7)装置F中装有淀粉I2水溶液,溶液显蓝色,SO2与I2的水溶液发生氧化还原反应,使溶液蓝色逐渐褪去,反应的离子方程式为SO2+I2+2H2O=SO42-+2I-+4H+。

 阅读快车系列答案
阅读快车系列答案