��Ŀ����
����Ŀ����ú��ʯ���п�������������ԭ��A��B��A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��һ�ֱ�ˮ�����״Һ̬����0.1 mol��������������������ȫȼ�գ�����0.6 mol CO2��0.3 molH2O���ش��������⣺
(1)A�ĵ���ʽ________��B�Ľṹ��ʽ________��
(2)��������A��B��ȫȼ��ʱ����O2�����ʵ���A_______B(������������������������)
(3)��A���ڵ�ͬϵ��Cʹ������Ȼ�̼��Һ��ɫ�Ļ�ѧ��Ӧ����ʽ��_________________����Ӧ���ͣ�______________��
���𰸡�![]()
![]() �� CH2=CHCH3��Br2��CH2BrCHBrCH3 �ӳɷ�Ӧ
�� CH2=CHCH3��Br2��CH2BrCHBrCH3 �ӳɷ�Ӧ
��������
A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪCH2=CH2��B��һ�ֱ�ˮ�����״Һ̬����0.1mol��������������������ȫȼ�գ�����0.6mol CO2��0.3molˮ��������N��C��= 0.6mol/0.1mol=6��N��H��= 0.3mol��2/0.1mol=6����B�ķ���ʽΪC6H6��B����Է�������Ϊ78����12n+2n-6=78�����n=6������BΪ����
��1��AΪ��ϩ���ṹ��ʽΪCH2=CH2������ʽΪ��![]() ��BΪ�����ṹ��ʽΪ
��BΪ�����ṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
��2����ϩ��HԪ�����������ȱ���HԪ��������������ͬ��������ϩ����ȼ�գ���ϩ���ĵ��������࣬����������A��B��ȫȼ��ʱ����O2�����ʵ���A��B���ʴ�Ϊ������
��3����A���ڵ�ͬϵ��CΪCH2=CHCH3��CH2=CHCH3����ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��CH2=CHCH3+Br2��CH2BrCHBrCH3���ʴ�Ϊ��CH2=CHCH3+Br2��CH2BrCHBrCH3���ӳɷ�Ӧ��

����Ŀ����ʹ������к͵ζ����ⶨ���۰״�(��Ҫ�ɷ���CH3COOH)��������(g��100 mL��1)����֪CH3COOH + NaOH===CH3COONa + H2O �յ�ʱ������Һ�ʼ��ԡ�
��.ʵ�鲽�裺
(1)����Һ����ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL__________(����������)�ж��ݣ�ҡ�ȼ��ô���״���Һ��
(2)��_____ȡ����״���Һ20.00 mL����ƿ�С�
(3)�μ�2��_____________��ָʾ����
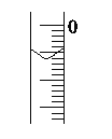
(4)��ȡʢװ0.100 0 mol��L��1NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ������ ���Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
(5)�ζ�����__________________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��.ʵ���¼
ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
V(��Ʒ) | 20.00 | 20.00 | 20.00 | 20.00 |
V(NaOH)(����) | 15.95 | 15.00 | 15.05 | 14.95 |
��.���ݴ��������ۣ�
(1)�����������ݷ�����c(���۰״�)��________mol��L��1��
(2)�ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫС����_______(��д���)��
A����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
B����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
C����ƿ�м������״���Һ���ټ�����ˮ
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
E. �ζ��յ����ʱ����