��Ŀ����
����Ŀ����֪A��B��C��D��E��F�����ڱ�ǰ�����ڵ�Ԫ�أ�ԭ��������������A��̬ԭ���У�����ռ�ݵ�����ܲ����ΪL������������ԳɶԵ��ӣ�����̬�����4���ɵ����ӣ�C�Ļ�̬ԭ���е���ռ�ݵ�����ܲ����ҲΪL��p�ܼ�����4�����ӣ�Dԭ�ӵ���������������Ӳ���֮������A��B��C����Ԫ�ص�ԭ������֮�ͣ�Eԭ�Ӻ��������������ԭ�Ӷ�������F��̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӡ���ش��������⣺
(1)��̬F��������Ų�ʽΪ______________________��
(2)��̬Eԭ�ӵļ۵����Ų�ʽΪ______________________��
(3)DC4���Ŀռ乹����___________����DC4����Ϊ�ȵ������һ�ַ���Ϊ___________(�ѧʽ)��HBC3���Ա�HBC2ǿ����ԭ����______________________��
(4)A��B��C����Ԫ�ص�һ������������___________(��Ԫ�ط��ű�ʾ)����ԭ����_________________________________��
(5)��E(NO3)2��Һ����μ��백ˮ���տ�ʼʱ������ɫ��E(OH)3����������ˮ����ʱ���������ܽ⣬����[E(NH3)6]2+����ɫ��Һ����1mol[E(NH3)6]2+���е�������ĿΪ___________��
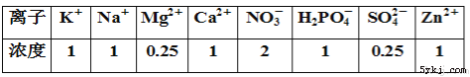
(6)D��Na�γɵ�һ�����ӻ��������ͼ��ʾ�����ṹͼ�к����ʾNa��λ�ã������ʾD��λ�ã���֪�þ����ı߳�Ϊncm�������ӵ�����ΪNA���������ܶ���=___________g/cm3(�ú�n��NA�ļ���ʽ��ʾ)��

���𰸡�1s22s22p63s23p63d104s1 3d84s2 �������� CCl4�� HNO3��2�����ǻ�������HNO2��1�����ǻ��� N �����̬����ϵ�������ϵͣ�ԭ���ȶ� 24NA 234/(n3NA)
��������
A��B��C��D��E��F�����ڱ�ǰ�����ڵ�Ԫ�أ�ԭ��������������A��̬ԭ���У�����ռ�ݵ�����ܲ����ΪL������������ԳɶԵ��ӣ�����̬�����4���ɵ����ӣ�A��C��C�Ļ�̬ԭ���е���ռ�ݵ�����ܲ����ҲΪL��p�ܼ�����4�����ӣ�C��O��A��B��C��ԭ��������������BΪN��Dԭ�ӵ���������������Ӳ���֮������A��B��C����Ԫ�ص�ԭ������֮�ͣ�D��Cl��Eԭ�Ӻ��������������ԭ�Ӷ�������E��Ni��F��̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�F��Cu��
��1��F��̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�F��ԭ����������E��F���ڵ������ڣ����������Ϊ2+8+18+1=29����FΪCu����������Ų�ʽΪ1s22s22p63s23p63d104s1���ʴ�Ϊ��1s22s22p63s23p63d104s1��
��2��E��Ni����̬Eԭ�ӵļ۵����Ų�ʽΪ3d84s2���ʴ�Ϊ��3d84s2��
��3��DC4����ClO4-��Clԭ�ӹµ��Ӷ���0���۲���Ӷ���4������ռ乹�������������ͣ���ClO4-��Ϊ�ȵ������һ�ַ��Ӻ���5��ԭ�ӡ�32���۵��ӣ��÷���ΪCCl4�ȣ�HBC3���Ա�HBC2ǿ��HNO3���Ա�HNO2ǿ������ΪHNO3��2�����ǻ�������HNO2��1�����ǻ������ʴ�Ϊ���������壻CCl4�ȣ�HNO3��2�����ǻ�������HNO2��1�����ǻ�����
��4��A��B��C�ֱ�ΪC��N��O����һ������������N����ԭ����N����Χ�����Ų�ʽΪ2s22p3��2p������ڰ����̬����ϵ�������ϵͣ�ԭ���ȶ����ʴ�Ϊ��N �������̬����ϵ�������ϵͣ�ԭ���ȶ���
��5��E(NO3)2ΪNi(NO3)2��1mol[Ni(NH3)6]2+���еĦҼ���ĿΪ24NA���ʴ�Ϊ��24NA��
��6��D��Cl�������������ӵ���ĿΪ4�������ӵ���ĿΪ4����������Ϊ[4��(58.5��NA)]g�������ı߳�ΪΪncm�������ܶ��Ǧ�=[4��(58.5��NA)]g��(ncm)3=234/(n3NA) g/cm3���ʴ�Ϊ��234/(n3NA)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�