��Ŀ����
����Ŀ��������һ�����͵���ɫ��Դ������һ����Ҫ�Ļ���ԭ�ϡ�
��1������ȼ����ֵ�ߡ�ʵ����,�ڳ��³�ѹ��1gH2��ȫȼ������Һ̬ˮ,�ų�142.9kJ���������ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ_____________________������֪��![]() ,�����ڿ�����ȼ������Һ̬ˮ�͵���ʱ���Ȼ�ѧ����ʽΪ______________________��
,�����ڿ�����ȼ������Һ̬ˮ�͵���ʱ���Ȼ�ѧ����ʽΪ______________________��
��2�������Ǻϳɰ�����Ҫԭ�ϡ�
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ�������(���ı�![]() ��
��![]() ����)����Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
����)����Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
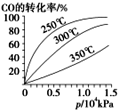
ͼ��t1ʱ����ƽ���ƶ�������������_______________,���б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����______________��
�ڰ����������������ᣬ�˹������漰���������![]() �ȡ����ڷ�Ӧ��
�ȡ����ڷ�Ӧ��![]() ,���¶�Ϊ
,���¶�Ϊ![]() ʱ,ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
ʱ,ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
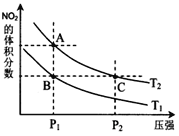
����˵����ȷ����______��
a��![]() ����Ļ�ѧƽ�ⳣ����
����Ļ�ѧƽ�ⳣ����![]()
b��![]() �����������ɫ��
�����������ɫ��![]() dz��
dz��![]() ��
��
c��![]() ���������ƽ����Է���������
���������ƽ����Է���������![]()
d��![]() ����ķ�Ӧ���ʣ�
����ķ�Ӧ���ʣ�![]()
e����״̬B��״̬A�������ü��ȵķ���
���𰸡�H2(g)+1/2O2(g)=H2O(l) H����285.8kJ/mol 4NH3(g)+3O2(g)=2N2(g)+6H2O(l) H����1530kJ/mol ��ѹ t2��t3 bde
��������
(1)1gH2��ȫȼ������Һ̬ˮ,�ų�142.9kJ��������1mol������2g������ȫȼ������Һ̬ˮ,�ų�142.9kJ��2��������˱�ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪH2(g)+1/2O2(g)=H2O(l) H����285.8kJ/mol �١�����Ϊ![]() �ڣ���˸��ݸ�˹���ɿ�֪�١�6���ڡ�2���õ�4NH3(g)+3O2(g)=2N2(g)+6H2O(l) H����1530kJ/mol��
�ڣ���˸��ݸ�˹���ɿ�֪�١�6���ڡ�2���õ�4NH3(g)+3O2(g)=2N2(g)+6H2O(l) H����1530kJ/mol��
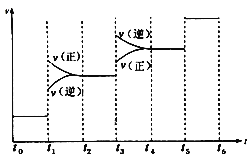
(2)������Ӧ�������С�ķ��ȷ�Ӧ����ͼ�������t1ʱ���淴Ӧ���ʾ���������Ӧ���ʴ����淴Ӧ���ʣ�������Ӧ������У����ƽ���ƶ��������Ǽ�ѹ��t3ʱ���淴Ӧ���ʾ������淴Ӧ���ʴ�������Ӧ���ʣ����淴Ӧ������У����ƽ���ƶ������������¡���ѹƽ�������ƶ���NH3�ĺ�����������ʱƽ�������ƶ���NH3�ļ�С�����Ա�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����t2��t3��
��a��![]() �����Ӧ���¶���ͬ����ѧƽ�ⳣ����ȣ�a����
�����Ӧ���¶���ͬ����ѧƽ�ⳣ����ȣ�a����
b��A�������ѹǿС��C�������ѹǿ��ѹǿ�������Ũ�ȴ���ɫ����������������ɫ��![]() dz��
dz��![]() ���ȷ��
���ȷ��
c����ͼ�п��Կ�����![]() ����NO2�����������ͬ������ƽ����Է���������ȣ�����
����NO2�����������ͬ������ƽ����Է���������ȣ�����
d��ѹǿA��С��C�㣬��������ķ�Ӧ���ʣ�![]() ����ȷ��
����ȷ��
e����״̬B��״̬A��NO2������������ӣ�ƽ��������Ӧ������У���˿����ü��ȵķ�������ȷ��
��Ϊbde��

����Ŀ��ij�о�С�齫������SO2���建����ͨ�뵽ʢ��25mL0.1mol�� L��1��Ba(NO3)2��Һ�У��õ�BaSO4������Ϊ̽���÷�Ӧ�е�����������С����������¼��裺
����������Һ�е�NO3����
��������________________��
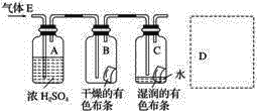
(1)��С�����������ʵ����֤�˼���������(Ϊ�ų��������Լ������ĸ��ţ�����������ʵ��������Һʱ��Ӧ___________________)������д�±���
ʵ�鲽�� | ʵ������ | ���� | |
ʵ���� | ��ʢ��25mL0.1mol��L��1BaCl2��Һ���ձ��л���ͨ�봿����SO2���� | ______ | ���������� |
ʵ���� | ��ʢ��25mL0.1mol�� L��1Ba(NO3)2��Һ���ձ��л���ͨ�봿����SO2���� | ______ | |
(2)Ϊ�����о��÷�Ӧ����С�黹�����������ʵ������Һ��pH��ͨ��SO2����ı仯������ͼ��V1ʱ��ʵ��������ҺpHС��ʵ������ԭ����(�����ӷ���ʽ��ʾ)��________��

(3)��֤��������ijͬѧ��������·�������������б���(���Բ�����)��
ʵ�鲽�� | ʵ������ | ʵ��Ŀ�� | ||
ʵ���� | ͬʵ�������� | ͬʵ������������� | ______ | |
ʵ���� | ______ | ______ | ______ | |
(4)������֪��H2SO3�Ƕ�Ԫ��(Kl=1.54��10��2��K2=1.02��10��7)�������ʵ�鷽����֤H2SO3�Ƕ�Ԫ��______(�Լ���������ѡ)��
����Ŀ��I������(H2C2O4)���������������������ܹ�������Ӧ��
��ʵ��1����ͬѧ��8.00 mL 0.001 mol/L KMnO4��Һ��5.00 mL 0.01 mol/L H2C2O4��Һ��Ӧ���о���ͬ�����Ի�ѧ��Ӧ���ʵ�Ӱ�졣�ı���������£�
��� | KMnO4��Һ /mL | H2C2O4��Һ /mL | 10%�������/mL | �¶�/�� | �������� |
�� | 8.00 | 5.00 | 3.00 | 20 | |
�� | 8.00 | 5.00 | 3.00 | 30 | |
�� | 8.00 | 5.00 | 1.00 | 20 | 2.00 mL����ˮ |
��1��д������(H2C2O4)����������Һ�����������·�Ӧ�����ӷ���ʽ________��
��2����������ʵ����Ŀ����̽��__________�Ի�ѧ��Ӧ���ʵ�Ӱ�졣
��ʵ��2����ͬѧ���о������������������������·�Ӧ��Ӱ������ʱ����,���������Ը��������Һ��ʼһ��ʱ�䷴Ӧ���ʽ���,��Һ��ɫ������,�����ú�ͻȻ��ɫ,��Ӧ�������Լӿ졣
��3�������������,��ͬѧ��Ϊ�����������ط�Ӧ����,������Һ�¶�����,��Ӧ���ʼӿ졣��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�,����뻹������_______��Ӱ�졣
��4������ʵ��֤����IJ���,�������Ը��������Һ�Ͳ�����Һ��,����Ҫѡ����Լ����������____������ĸ����
a������� b��ˮ c���������� d��������
������ͼ��ʾ��װ�ý����к��ȵIJⶨʵ�飬�ֱ�ȡ![]() ��
��![]() ��Һ��
��Һ��![]() ���������ʵ�飬�ش��������⣺
���������ʵ�飬�ش��������⣺
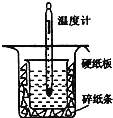
��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��__________������֮�⣬װ���е�һ�����Դ�����__________��
��2��������Ϊ![]() ��NaOH��Һ��
��NaOH��Һ��![]() ��������Һ���ܶȶ���
��������Һ���ܶȶ���![]() ���кͺ�������Һ�ı�����
���кͺ�������Һ�ı�����![]() ��ͨ���������ݼ����к��ȡ�H=__________���������С�����һλ����
��ͨ���������ݼ����к��ȡ�H=__________���������С�����һλ����
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | ||
H2SO4 | NaOH | ƽ��ֵ | ||
1 | 26.2 | 26.0 | 26.1 | 29.5 |
2 | 27.0 | 27.4 | 27.2 | 32.3 |
3 | 25.9 | 25.9 | 25.9 | 29.2 |
4 | 26.4 | 26.2 | 26.3 | 29.8 |
��3������ʵ����ֵ�����![]() ��ƫ�����ƫ���ԭ������ǣ�����ĸ��_____��
��ƫ�����ƫ���ԭ������ǣ�����ĸ��_____��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨ![]() ��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�![]() ��Һ����ʢ�������С�ձ���
��Һ����ʢ�������С�ձ���
d����������ʵ������¶Ⱦ��������ƽ��ֵ