��Ŀ����
����Ŀ��NԪ�صĵ��ʼ��仯����֮���ת����ϵ��ͼ��ʾ��NH3��N2��NO��NO2��HNO3
�ش��������⣺
��1��NԪ����Ԫ�����ڱ��е�λ��Ϊ��___���ڣ���___�壻����Nԭ�ӽṹʾ��ͼ___��
��2����ҵ���õ����������ϳɰ�������д��NH3�ĵ���ʽ___��
��3��ʵ���ҿ��������������ƹ�����Ȼ�粒����Ʊ���������д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ___����д��ʵ���Ҽ��鰱���ľ������___��
��4��NO��������Ѫ�ܼ�������ʹ���ӣ�NO������Ӵ��ķ�Ӧ������___��д���÷�Ӧ�Ļ�ѧ����ʽ___�����÷�Ӧת��0.4mole-�������ĵ�NO��������___����NA��ʾ����٤����������
��5��Ũ������в��ȶ��ԣ�ʵ����Ӧ�ý�Ũ���ᱣ���ڴ���������___��������ɫ��������ɫ�����Լ�ƿ�С�
���𰸡��� ��A ![]()
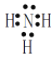 2NH4Cl��Ca(OH)2
2NH4Cl��Ca(OH)2![]() CaCl2��2H2O��2NH3�� ��ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ�����ֽ��������˵�����ɰ��� �����Ϊ����ɫ
CaCl2��2H2O��2NH3�� ��ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ�����ֽ��������˵�����ɰ��� �����Ϊ����ɫ ![]() 0.2NA ��ɫ
0.2NA ��ɫ
��������
��1��Nԭ����2�����Ӳ㣬�����5�����ӣ�
��2��NH3�е�ԭ��ͨ����������ԭ�ӽ�ϣ�
��3���������ƹ�����Ȼ�粒��干�������Ȼ��ơ�������ˮ��������ʹʪ��ĺ�ɫʯ����ֽ������
��4��NO��������Ӧ���ɺ���ɫNO2��
��5��Ũ������в��ȶ��ԣ������ֽ⣻
��1��Nԭ����2�����Ӳ㣬�����5�����ӣ�����NԪ����Ԫ�����ڱ��е�λ��Ϊ�ڶ����ڣ�����A�壻 Nԭ�ӽṹʾ��ͼ��![]() ��
��
��2��NH3�е�ԭ��ͨ����������ԭ�ӽ�ϣ�NH3�ĵ���ʽ��![]() ��
��
��3���������ƹ�����Ȼ�粒��干�������Ȼ��ơ�������ˮ����Ӧ����ʽ��2NH4Cl��Ca(OH)2![]() CaCl2��2H2O��2NH3������ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ�����ֽ��������˵�����ɰ�����
CaCl2��2H2O��2NH3������ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ�����ֽ��������˵�����ɰ�����
��4��NO������Ӵ��ķ�Ӧ�����������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��![]() ��NԪ�ػ��ϼ���+2����Ϊ+4,ת��0.4mole-�����ĵ�0.2molNO����������0.2NA��
��NԪ�ػ��ϼ���+2����Ϊ+4,ת��0.4mole-�����ĵ�0.2molNO����������0.2NA��
��5��Ũ������в��ȶ��ԣ������ֽ⣬Ҫ�ܹⱣ�棬����ʵ���ҽ�Ũ���ᱣ���ڴ�����������ɫ�Լ�ƿ�С�