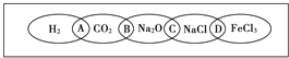
��Ŀ����
����Ŀ����ҵ�����õ�ⱥ��ʳ��ˮ��������(Cl2)���ռ�������Ļ�ѧ����ʽ��:2NaCl + 2H2O![]() NaOH + Cl2��+ H2��
NaOH + Cl2��+ H2��
(1)��˫���ű�ʾ������ת�Ƶķ������Ŀ______________����з�Ӧ��д�����ӷ���ʽ��___________________________________
(2)���ʳ��ˮ�Ĺ����У�����������__________________________________
(3)���ʳ��ˮҪ��Դ�ʳ��ˮ���о��ƣ��Գ�ȥ��ʳ��ˮ�к��е���ɳ��SO42-��Ca2+,Mg2+���������ӡ�����ʱ���μ����Ȼ�����Һ������ռ��ַ�Ӧ����ˣ�����Һ�м��������к������ԡ���������Һ��Ӧ�����ӷ���ʽ:___________________,________________________________.
(4)ʵ�������ù���NaOH������90ml 0.5 mol/L��NaOH��Һ���������ϳ��ӣ���Ҫ��������NaOH_______g������ʱ��һ��ɷ�Ϊ���¼������裺�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ������ȷ�IJ���˳��Ϊ__________�������ƹ����У���������������ȷ�����в�����������ƫ�͵���___________(����ĸ)��
A��û��ϴ���ձ��Ͳ�����
B��δ��NaOH��Һ��ȴ�����¾�ת�Ƶ�����ƿ�ж���
C������ƿ�����������������ˮ
���𰸡�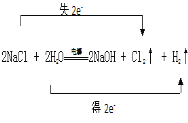 2Cl��+2H2O
2Cl��+2H2O![]() 2OH��+Cl2��+H2�� Cl2 H+ +OH��=H2O CO3 2- + 2H+ =H2O+ CO2�� 2.0 �ڢ٢ۢ�ݢޢߢ� A
2OH��+Cl2��+H2�� Cl2 H+ +OH��=H2O CO3 2- + 2H+ =H2O+ CO2�� 2.0 �ڢ٢ۢ�ݢޢߢ� A
��������
��1����Ӧ���Ȼ�������Ԫ�صĻ��ϼ����������������Ȼ�������ԭ����ˮ����Ԫ�ػ��ϼ۽�������������ˮ������������Ӧ��ת�Ƶ�����ĿΪ2e����
��2����Ӧ��ClԪ�ػ��ϼ����ߵı�����������Ϊ����������
��3������Һ�м������ᣬĿ���dz�ȥ��Һ�й����������Ӻ�̼���������
��4������n=cM������������Ƶ����ʵ�������������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���������������������ʵ����ʵ��������Һ�������Ӱ�죬����c=n/V�ж���
��1����ⱥ��ʳ��ˮ����������������Cl2�����ռ��Ӧ���Ȼ�������Ԫ�صĻ��ϼ����������������Ȼ���ʧ��������ԭ����ˮ����Ԫ�ػ��ϼ۽�������������ˮ�õ���������������Ӧ��ת�Ƶ�����ĿΪ2e����˫����Ϊ�� �����ӷ���ʽΪ��2Cl��+2H2O
�����ӷ���ʽΪ��2Cl��+2H2O![]() 2OH��+Cl2��+H2�����ʴ�Ϊ��
2OH��+Cl2��+H2�����ʴ�Ϊ�� ��2Cl��+2H2O
��2Cl��+2H2O![]() 2OH��+Cl2��+H2����
2OH��+Cl2��+H2����
��2����Ӧ��ClԪ�ػ��ϼ����ߵı��������Ȼ���ʧ��������ԭ��������Ϊ��������ʴ�Ϊ��Cl2��
��3������ʱ���μ����Ȼ�����Һ������ռ�Ȼ�����Һ��ȥ��������ӣ������ȥ�����ӡ������ӣ��ռ��ȥþ���ӣ���ַ�Ӧ����ˣ�����Һ�м������ᣬĿ���dz�ȥ��Һ�й����������Ӻ�̼������ӣ�������Һ�м��������к������Ե����ӷ���ʽΪ��H++OH-=H2O��CO3 2- + 2H+ =H2O+ CO2�����ʴ�Ϊ��H++OH-=H2O��CO3 2- + 2H+ =H2O+ CO2����
��4��ʵ��������90ml 0.5 mol/L��NaOH��ҺӦѡ��100ml����ƿ�����������Ƶ�����Ϊm=0.5L��0.1molL-1��40g/mol=2.0g���ʴ�Ϊ��2.0g��
���������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ����ʵ���Ⱥ�˳��Ϊ�ڢ٢ۢ�ݢޢߢܣ��ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
A����ϴ���ձ��Ͳ��������������ʲ�����ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ����
B��Һ������������������ʣ����������ܽ���ȣ�δ��ȴ�����£����Ƚ���Һ��������ƿ���������Һ����ȴ��ᵼ����Һ���ƫС����ҺŨ��ƫ����
C������ƿ�����������������ˮ�������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ�����
�ʴ�Ϊ��A��

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�����Ŀ��������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJmol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJmol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJmol-1 | K3 |
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g)![]() CH3OCH3(g)��CO2(g) ��H
CH3OCH3(g)��CO2(g) ��H
�÷�Ӧ��H��__________________����ѧƽ�ⳣ��K��____________________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g) ![]() CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч���� D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����_______________________________��