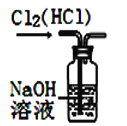
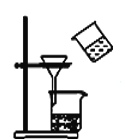
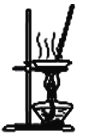
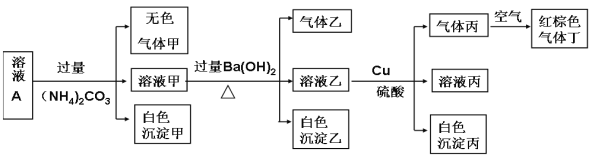
��Ŀ����
����Ŀ�������£����и���������ָ����Һ��һ���ܴ����������(����)
A. 1.0 mol��L��1 KNO3��Һ��H����Fe2����SCN����SO42��
B. c(H��)/c (OH��)��10��10����ɫ��Һ��Na ����Ba2����NO3����Cl��
C. ��ˮ�������c(H��)��10��10mol��L��1����Һ��![]() ��AlO2����Ca2����S2��
��AlO2����Ca2����S2��
D. c(ClO��)��1.0 mol��L��1����Һ��K����SO32����S2����SO42��
���𰸡�B
��������
A��1.0molL-1KNO3��Һ�д��ڴ�����������ӣ�����������ڴ���H+�����������ܹ�����Fe2+���ӣ�����Һ�в��ܴ������棬��A���� B�����ݳ�����ˮ�����ӻ�����֪��������Һ������������Ũ��Ϊ0.01mol/L��Na+��Ba2+��NO3-��Cl-����֮�䲻������Ӧ���Ҷ��������������ӷ�Ӧ������Һ���ܹ��������棬��B��ȷ��C����ˮ�������c��H+��=10-10mol/L����Һ�д��ڴ���H+��OH-��NH4+��Ca2+�������������ӷ�Ӧ��S2-��AlO2-���������ӷ�Ӧ��Ca2+��S2-��Ӧ������Һ��һ�����ܴ������棬��C����D����Һ��ClO-�ܹ�����SO32-��S2-������Һ�в��ܴ������棬��D����ѡB��

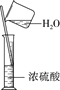
����Ŀ���������ʵ����ѡ���װ�û�����(�г�װ������ȥ)��ȷ����(����)
|
|
A.��CCl4��ȡ��ˮ�е�Br2 | B.��KI��I2�Ĺ��������л���I2 |
|
|
C.ϡ��Ũ���� | D.��ҵ�ƾ��Ʊ���ˮ�ƾ� |
A. A B. B C. C D. D